1 M NaOH solution was slowly added in to 1000 mL of 183.75 g impure H2SO4 solution and the following plot was obtained. The percentage purity of H2SO4 sample and slope of the curve respectively are: 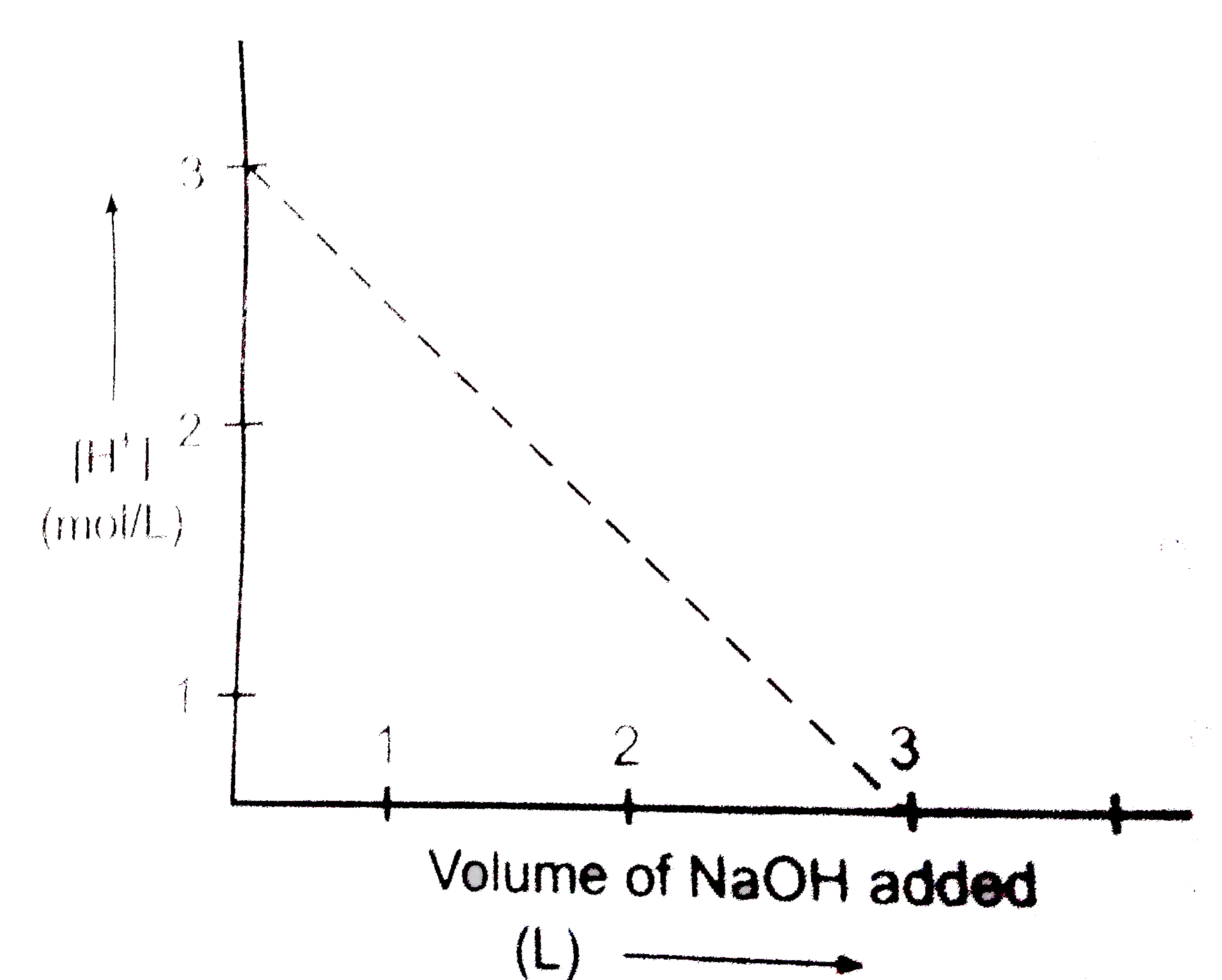
75%, −13
80%, −12
80%, -1
None of these
The correct Answer is:C
Step by step video, text & image solution for 1 M NaOH solution was slowly added in to 1000 mL of 183.75 g impure H_(2)SO_(4) solution and the following plot was obtained. The percentage purity of H_(2)SO_(4) sample and slope of the curve respectively are: by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
1 M NaOH solution was slowly added to 1 L of 210 g impure H2SO4 solution and the following plot was otained. Calculate the percentage purity of H2SO4 sample.
View Solution100 ml of 0.5 N NaOH solution is added to 10 ml of 3NH2SO4 solution and 20 ml of 1 N HCl solution. The mixture is __________
View Solution
Knowledge Check
1 M NaOH solution was slowly added in to 1000 mL of 183.75 g impure H2SO4 solution and the following plot was obtained. The percentage purity of H2SO4 sample and slope of the curve respectively are:
 A75%B60%C80%DNone of these
A75%B60%C80%DNone of theseTo 1L of 1.0M impure H2SO4 sample, 1.0MNaOH solution was added and a plot was obtained as follows:
The % purity of H2SO4 and the slope of curve, respectively, are:
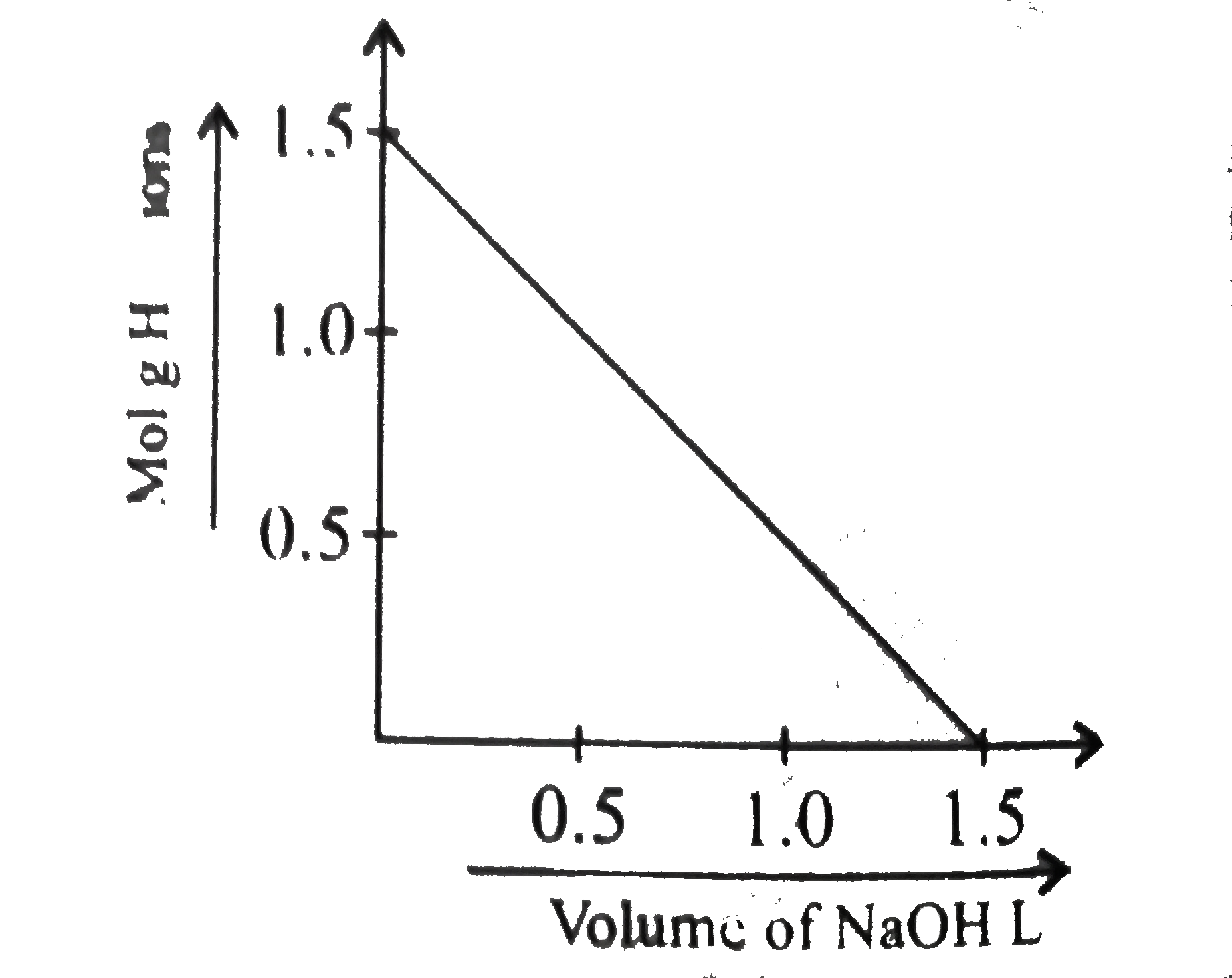 A75%,−1/2B75%,−1C50%,−1/3D50%,−1/2
A75%,−1/2B75%,−1C50%,−1/3D50%,−1/2100 mL of 0.5 N NaOH solution is added to 10 mL of 3 N H2SO4 solution and 20 mL of 1 N HCl solution . The mixture is
AacidicBalkalineCneutralDnone of these
What volume of 96% H2SO4 solution (density 1.83 g/mL) is required to prepare 4 litre of 3.0 M H2SO4 solution ?
View SolutionWhen 100 ml 1N-NaOH solution and 10 ml of 10 N sulphuric acid solution are mixed together, the resulting solution will be
View Solution100mL of 0.1 M NaOH solution is titrated with 100mL of 0.5 M H2SO4 solution. The pH of the resulting solution is :( For H2SO4,Ka1=10−2)
View Solution0.80g of impure (NH4)2SO4 was boiled with 100mL of a 0.2N NaOH solution was neutralized using 5mL of a 0.2NH2SO4 solution. The percentage purity of the (NH4)2SO4 sample is:
View Solution0.7g of (NH4)2SO4 sample was boiled with 100mL of 0.2 N NaOH solution was diluted to 250 ml. 25mL of this solution was neutralised using 10mL of a 0.1 N H2SO4 solution. The percentage purity of the (NH4)2SO4sample is :
View Solution
