The energy of a I,II and III energy levels of a certain atom are E,4E3 and 2E respectively. A photon of wavelength λ is emitted during a transition from III to I. what will be the wavelength of emission for II to I?
λ2
λ
2λ
3λ
Step by step video & image solution for The energy of a I,II and III energy levels of a certain atom are E,(4E)/(3) and 2E respectively. A photon of wavelength lambda is emitted during a transition from III to I. what will be the wavelength of emission for II to I? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
The following figure shows the energy levels of a particular atom. When the system moves from 2E level to E level then lambda A photon of wavelength A is emitted. (4E)/3 The wavelength of the photon produced during the transition from level to E is
View SolutionThe given diagram indicates the energy levels of a certain atom. When the system moves from 2E level to E, a photon of wavelength 2 is emitted. The wavelength of photon produced during its transition from 4E/3 level of E is:
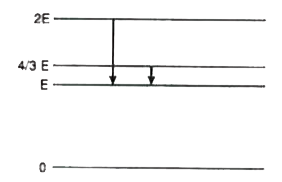
View SolutionThe energy of a I,II and III energy levels of a certain atom are E,4E3 and 2E respectively. A photon of wavelength λ is emitted during a transition from III to I. what will be the wavelength of emission for II to I?
View SolutionThe energy of a I, II and III energy levels of a certain atom are E, (4E)/(3) and 2E respectively A photon of wavelength lambda is emitted during a transition from III to I. What will be the wavelength of emission for transition II to I?
View SolutionThe follwing diagram indicates the energy levels of a certain atom when the system moves from 2E level to E, a photon of wavelength λ is emitted. The wavelength of photon produced during its transition from 4E3 level to E is
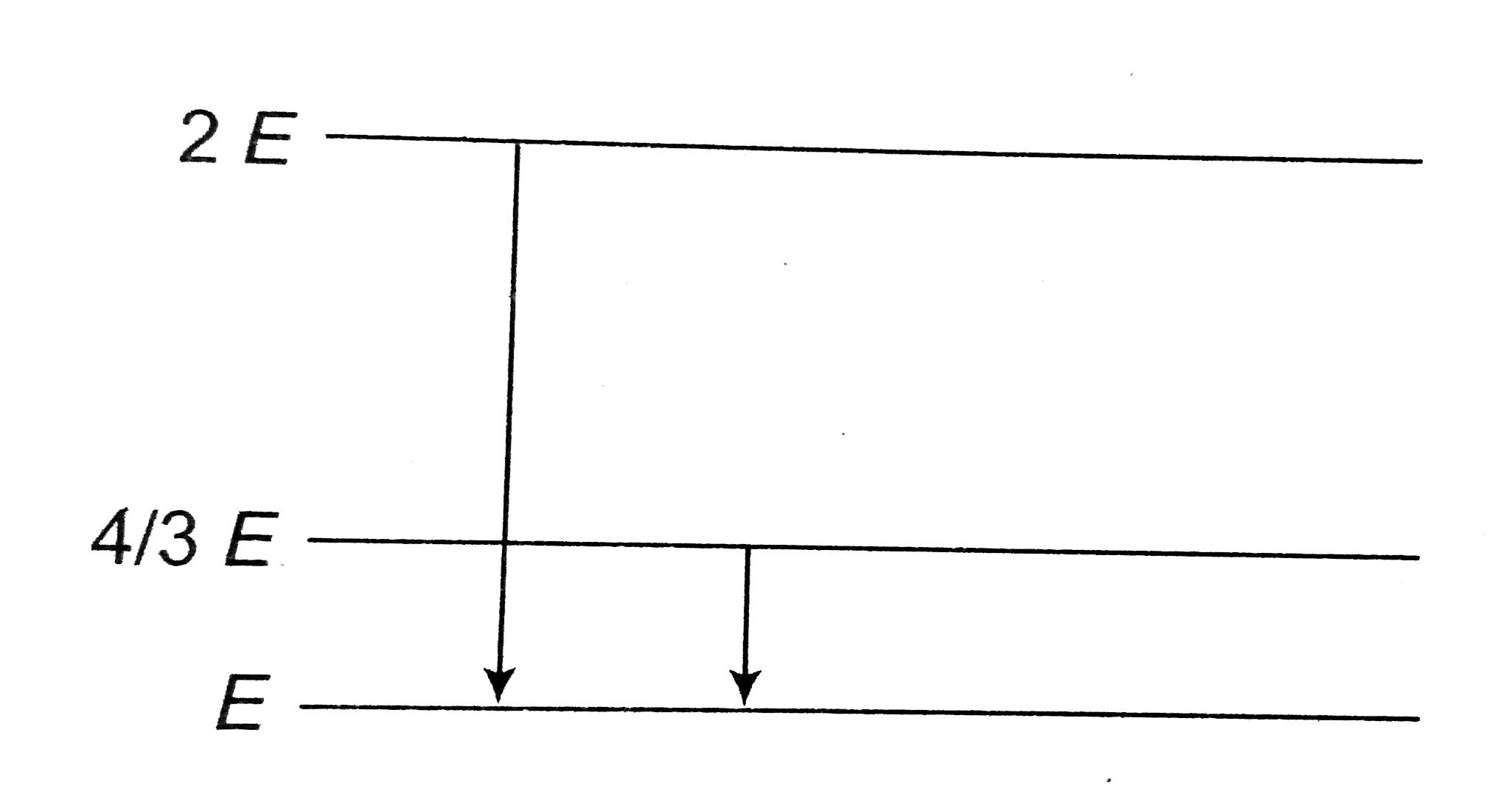
View SolutionThe energy of a I,II and III energy levels of a certain atom are E,4E3 and 2E respectively. A photon of wavelength λ is emitted during a transition from III to I. what will be the wavelength of emission for II to I?
View SolutionThe given diagram indicates the energy levels of a certain atom. When the system moves from 26 level to E level, a photon of wavelength 2 is emitted. The wavelength of the photon emitted during its transition from 4E/ 3 level to E level is
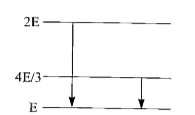
View SolutionThe follwing diagram indicates the energy levels of a certain atom when the system moves from 2E level to E , a photon of wavelength lambda is emitted. The wavelength of photon produced during its transition from (4E)/(3) level to E is
View Solution
