Consider the reaction 2CO(g)+O2(g)⇔2CO2(g)+Heat
Under what conditions shift is undetminable ?
Addition of O2 and decrease in volume
Addition of CO and removal of CO2 at constant volume
Increase in temperature and decrease in volume
Addition of CO and increase in temperature at constant volume
Step by step video & image solution for Consider the reaction 2CO(g)+O_(2)(g)hArr2CO_(2)(g)+Heat Under what conditions shift is undetminable ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
For a reaction C(s)+CO2(g)⇔2CO(g)Δn value is
AThe equlibrium shifts in backward direction if more of CO is addedBThe equilibrium shifts in backward direction if more of CO is addedCThe equlibrium shifts in forward direction if more of corbon is addedDOn adding carbon, the equlibrium is not disturbedFor the reaction, CO(g)+1/2O2(g)→CO2(g)
AΔH=ΔEBΔH>ΔECΔH<ΔEDΔH−ΔE=0Kp/Kc for the reaction
CO(g)+12O2(g)⇔CO2(g) isARTB(RT)1/2C1(RT)3D1√RT
For the reaction
CO(g)+12O2(g)⇔CO2(g),Kp/Kc is
View SolutionKp/Kc for the reaction
CO(g)+12O2(g)⇔CO2(g) is
View SolutionC(s)+CO2(g)⇔2CO(g)− Heat. The favourable conditions for the above reaction are
View SolutionThe energy profile diagram for the reaction:
CO(g)+NO2(g)⇔CO2(g)+NO(g) is given below:
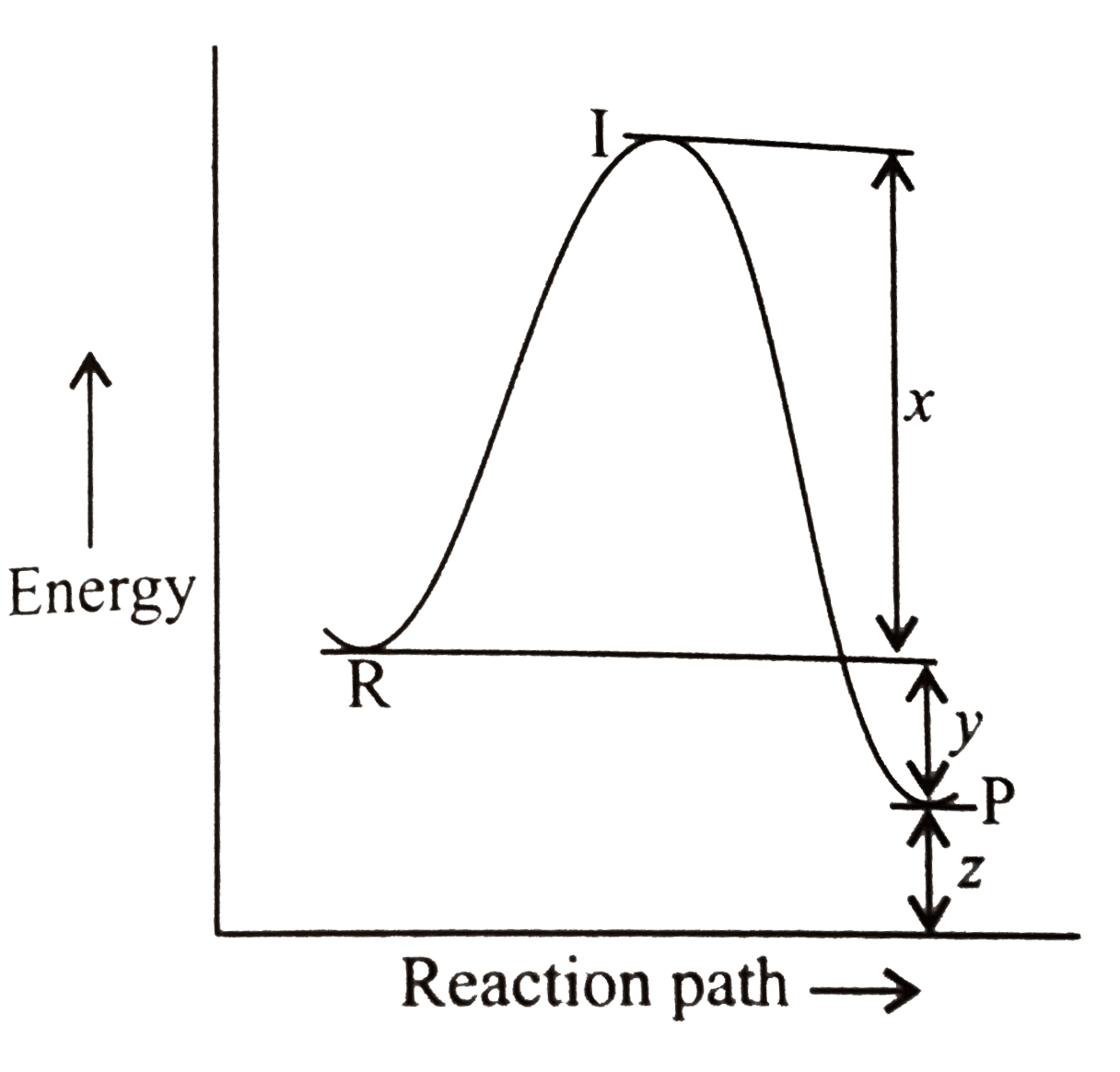
the heat of the reaction is
View SolutionFor the reaction
CO(g)+H2O(g)⇔CO2(g)+H2(g)
at a given temperature the equilibrium amount of CO2
(g) can be increased by
View Solution
