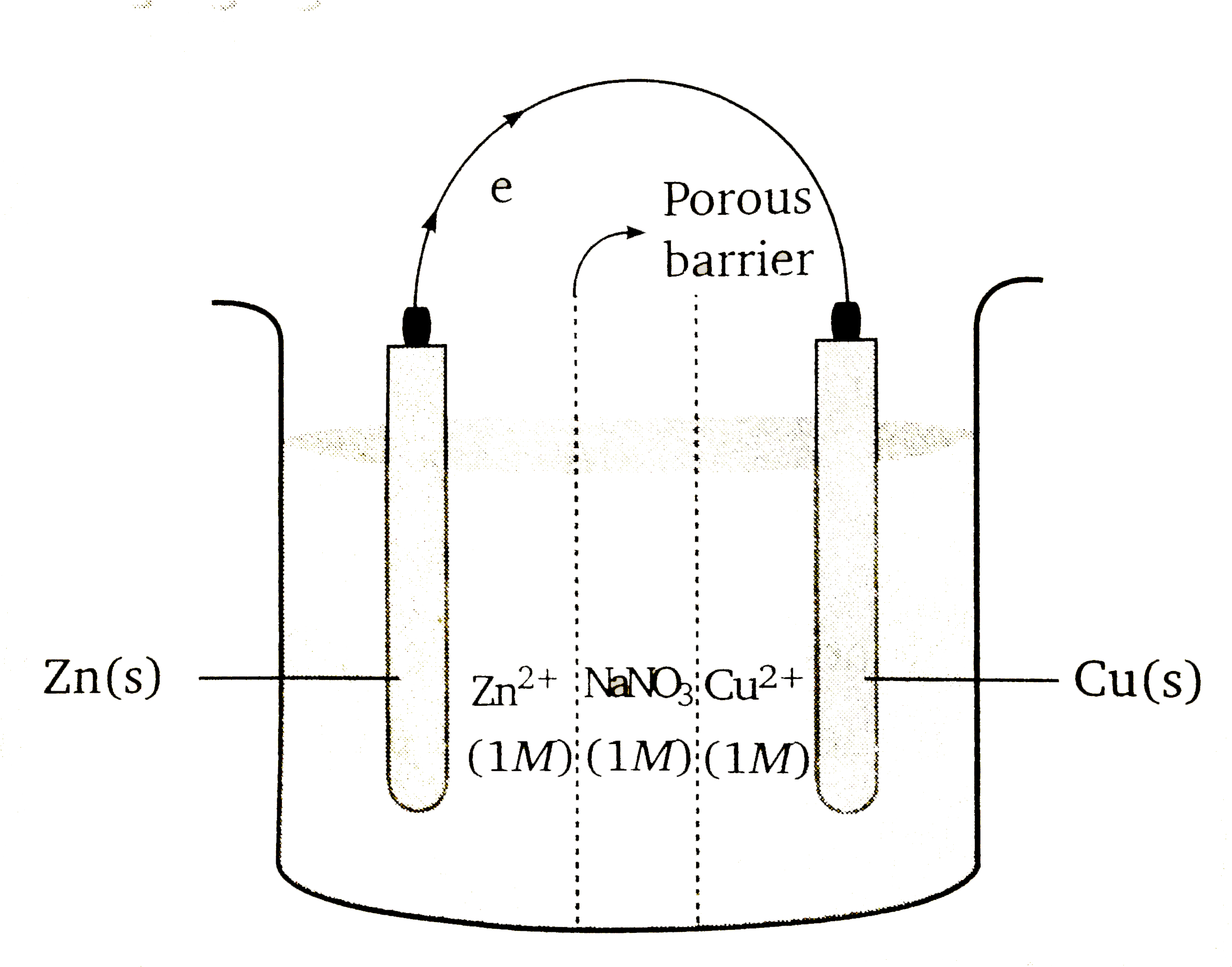
A Galvanic cell consits of three compartment as shown in figure. The first compartment contain ZnSO4(1M) and III compartment contain CuSO4(1M). The mid compartment contain NaNO3 (1M). Each compartment contain 1L solution:
E∘Zn2+/Zn=−0.76, E∘Cu2+/Cu=+0.34,
The concertation of Zn2+ in first compartment after passage of 0.1 F charge will be:
1M
1.05M
1.025M
0.5M
The correct Answer is:C
Similar Questions
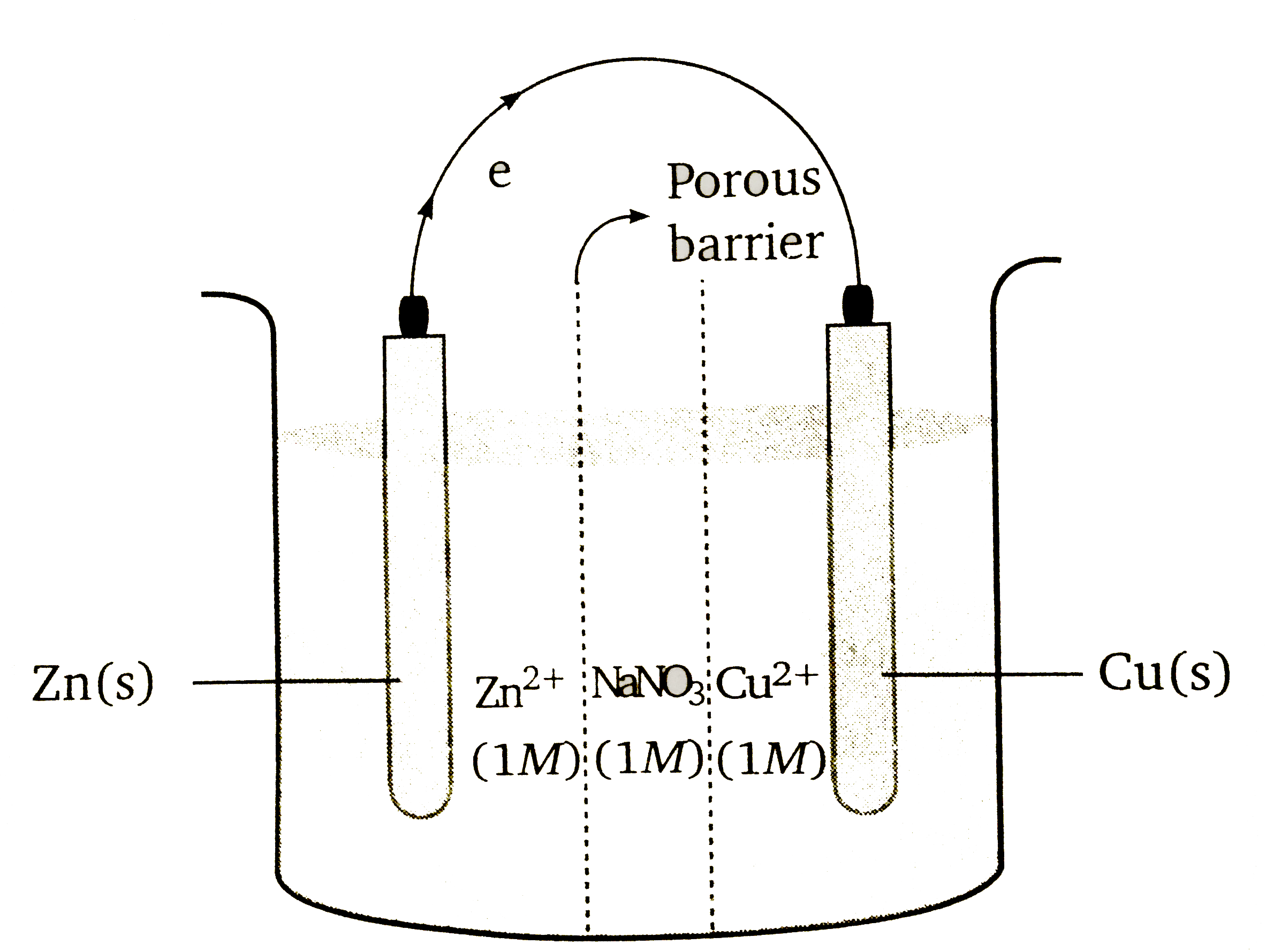
A Galvanic cell consits of three compartment as shown in figure. The first compartment contain ZnSO4(1M) and III compartment contain CuSO4(1M). The mid compartment contain NaNO3 (1M). Each compartment contain 1L solution:
E∘Zn2+/Zn=−0.76, E∘Cu2+/Cu=+0.34
The concentration of SO2−4 ions in III compartment after passage of 0.1 F of charge will be:
View Solution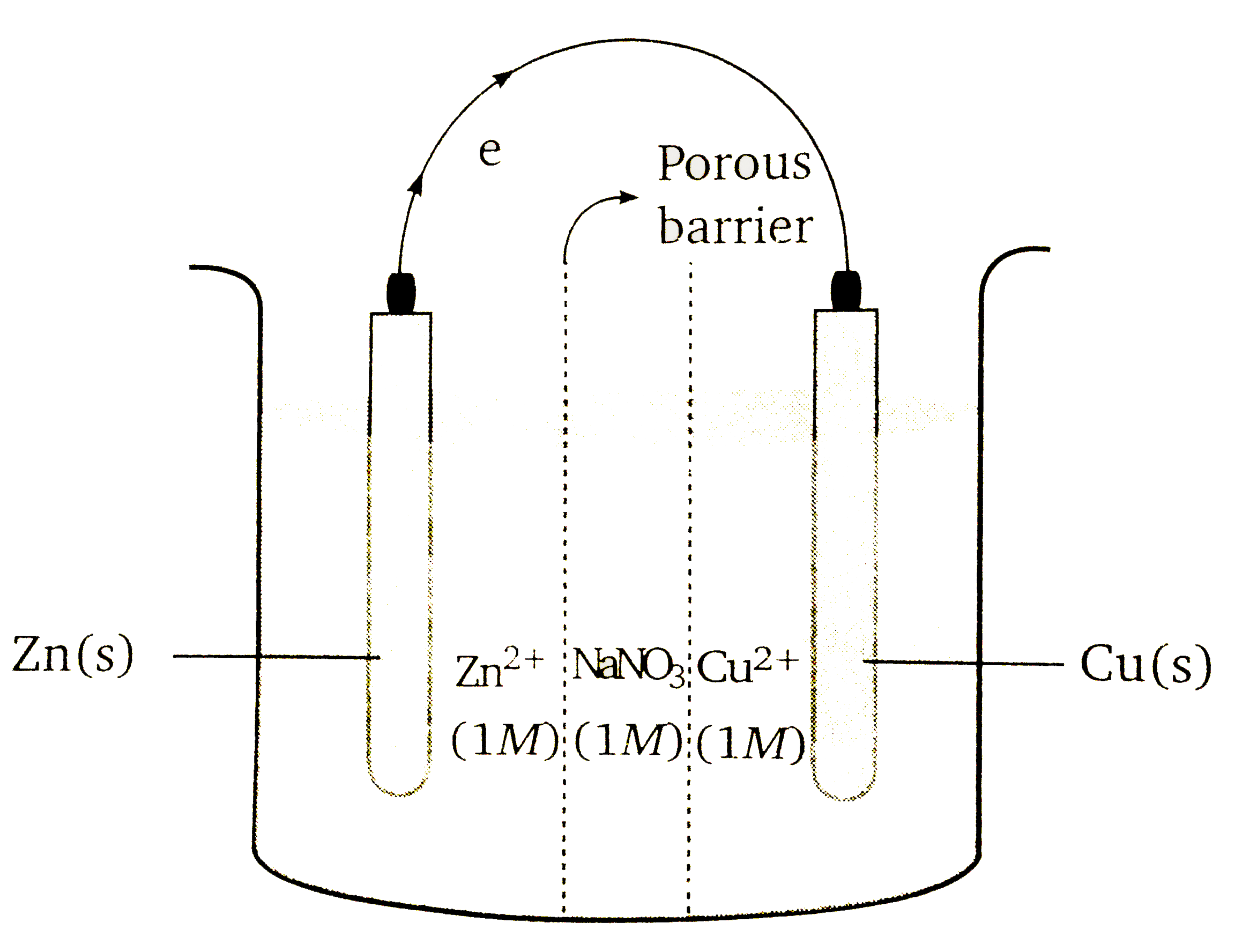
A Galvanic cell consits of three compartment as shown in figure. The first compartment contain ZnSO4(1M) and III compartment contain CuSO4(1M). The mid compartment contain NaNO3 (1M). Each compartment contain 1L solution:
E∘Zn2+/Zn=−0.76, E∘Cu2+/Cu=+0.34
The concentration of NO−3 in mid compartment after passage of 0.1 F of charge will be:
View Solution
Knowledge Check

A Galvanic cell consits of three compartment as shown in figure. The first compartment contain ZnSO4(1M) and III compartment contain CuSO4(1M). The mid compartment contain NaNO3 (1M). Each compartment contain 1L solution:
E∘Zn2+/Zn=−0.76, E∘Cu2+/Cu=+0.34
The concentration of SO2−4 ions in III compartment after passage of 0.1 F of charge will be:A1.05MB1.025MC0.95MD0.975M
A Galvanic cell consits of three compartment as shown in figure. The first compartment contain ZnSO4(1M) and III compartment contain CuSO4(1M). The mid compartment contain NaNO3 (1M). Each compartment contain 1L solution:
E∘Zn2+/Zn=−0.76, E∘Cu2+/Cu=+0.34
The concentration of NO−3 in mid compartment after passage of 0.1 F of charge will be:A0.95MB0.90MC0.975MD1.05MThe emf of the cell, (EZn2+/Zn=−0.76V)Zn/Zn2+(1M)∣∣Cu2+(1M)Cu
(ECu2+/Cu=+0.34V) will be :A+1.10VB−1.10VC+0.42VD−0.42V
Can 1 M ZnSO4 be stored in a vessel made up of copper ?
Given : E∘Zn2+/Zn=−0.76 and E∘Cu2+/Cu=+0.34 V?
View SolutionCan a solution of 1 M ZnSO4 be stored in a vessel made of copper ? Given that
E∘Zn+2/Zn=−0.76VandE∘Cu+2/Cu=0.34V
View SolutionCan a solution of 1 M ZnSO4 be stored in a vessel made of copper ? Given that
E∘Zn+2/Zn=−0.76VandE∘Cu+2/Cu=0.34V
View SolutionOut of Zn and Cu vessel one will be more suitable to store 1 M HCl?
Given EθZn2+/Zn=−0.76V,EθCu2+/Cu=+0.34V.
View SolutionThe emf of the cell, (EZn2+/Zn=−0.76V)Zn/Zn2+(1M)∣∣Cu2+(1M)Cu
(ECu2+/Cu=+0.34V) will be :
View Solution

