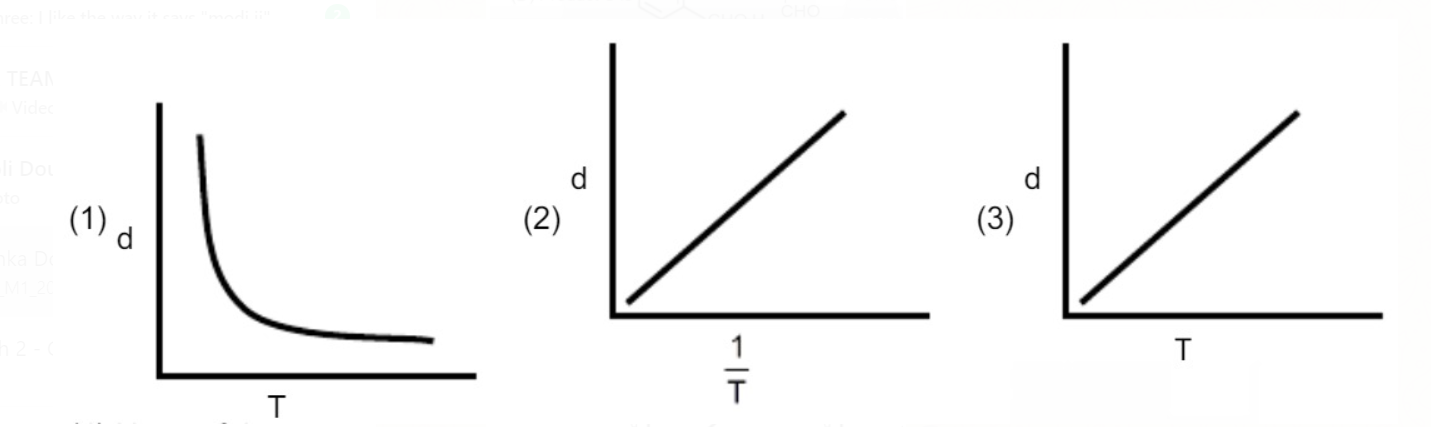
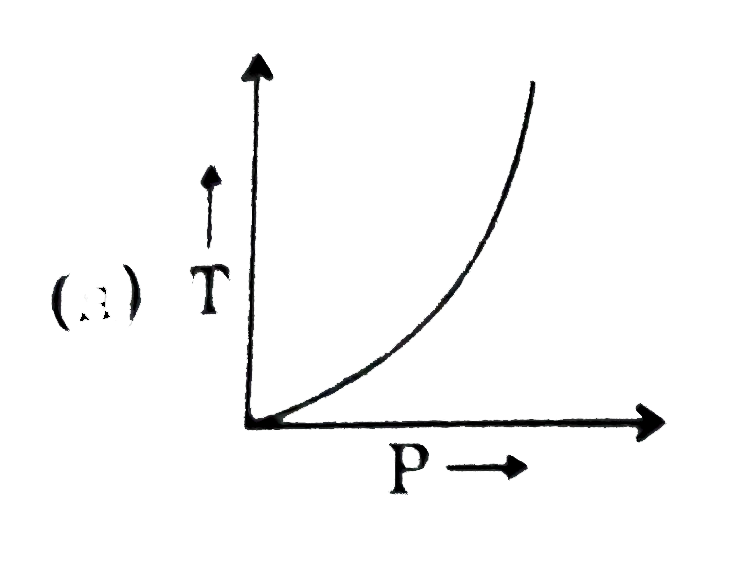
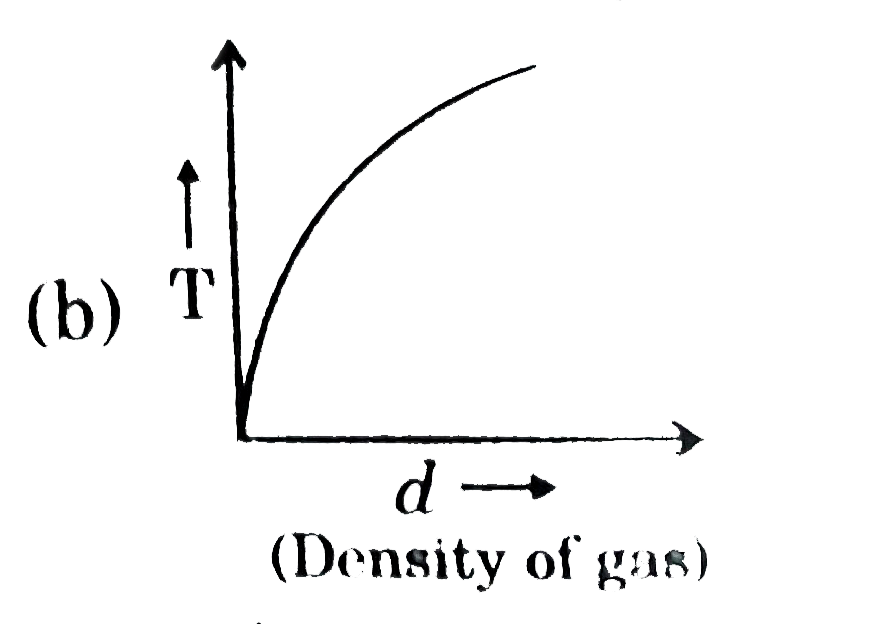
Which of the following graph is correct reversible adiabatic process for an ideal gas:
A
B
C
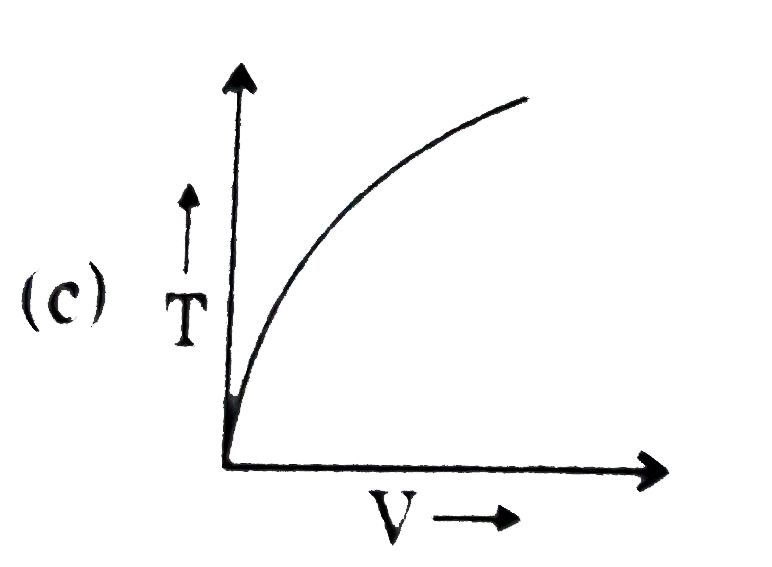
D
Video Solution
Text Solution
The correct Answer is:B
|
Answer
Step by step video, text & image solution for Which of the following graph is correct reversible adiabatic process for an ideal gas: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Which of the following is true for reversible adiabatic process involving an ideal gas ?
AGas with higherγ has high magnitude of slope in a P(y-axis)v/sT(x - axis) curveBGas with higherγ has high magnitude of slope in a V(y-axis)v/sT(x - axis) curveCGas with higherγ has high magnitude of slope in a P(y-axis)v/sV(x - axis) curveDGas with higherγ has low magnitude of slope in a P(y-axis)v/sT(x - axis) curveWhich of the following is correct regarding adiabatic process
A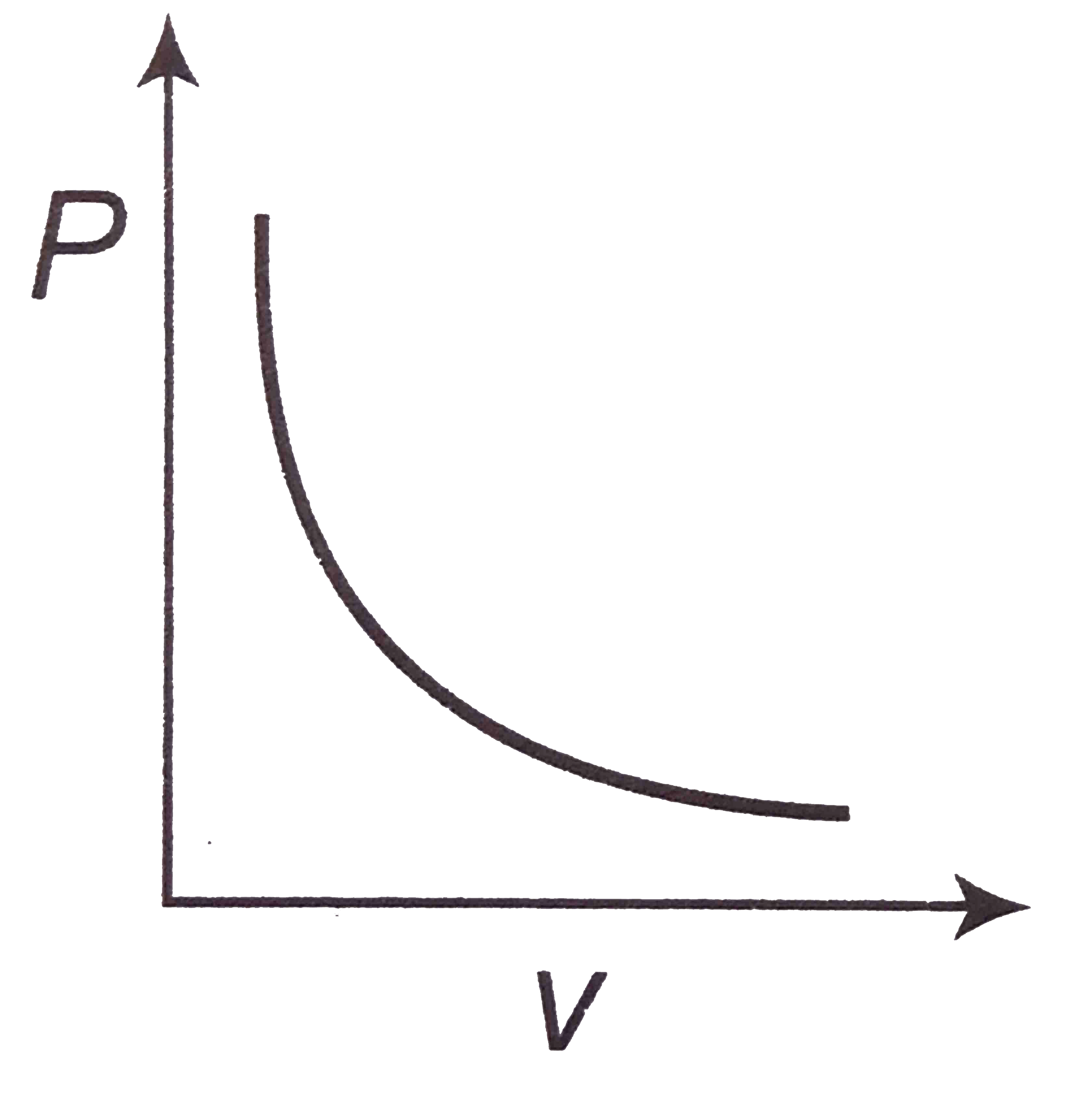 B
B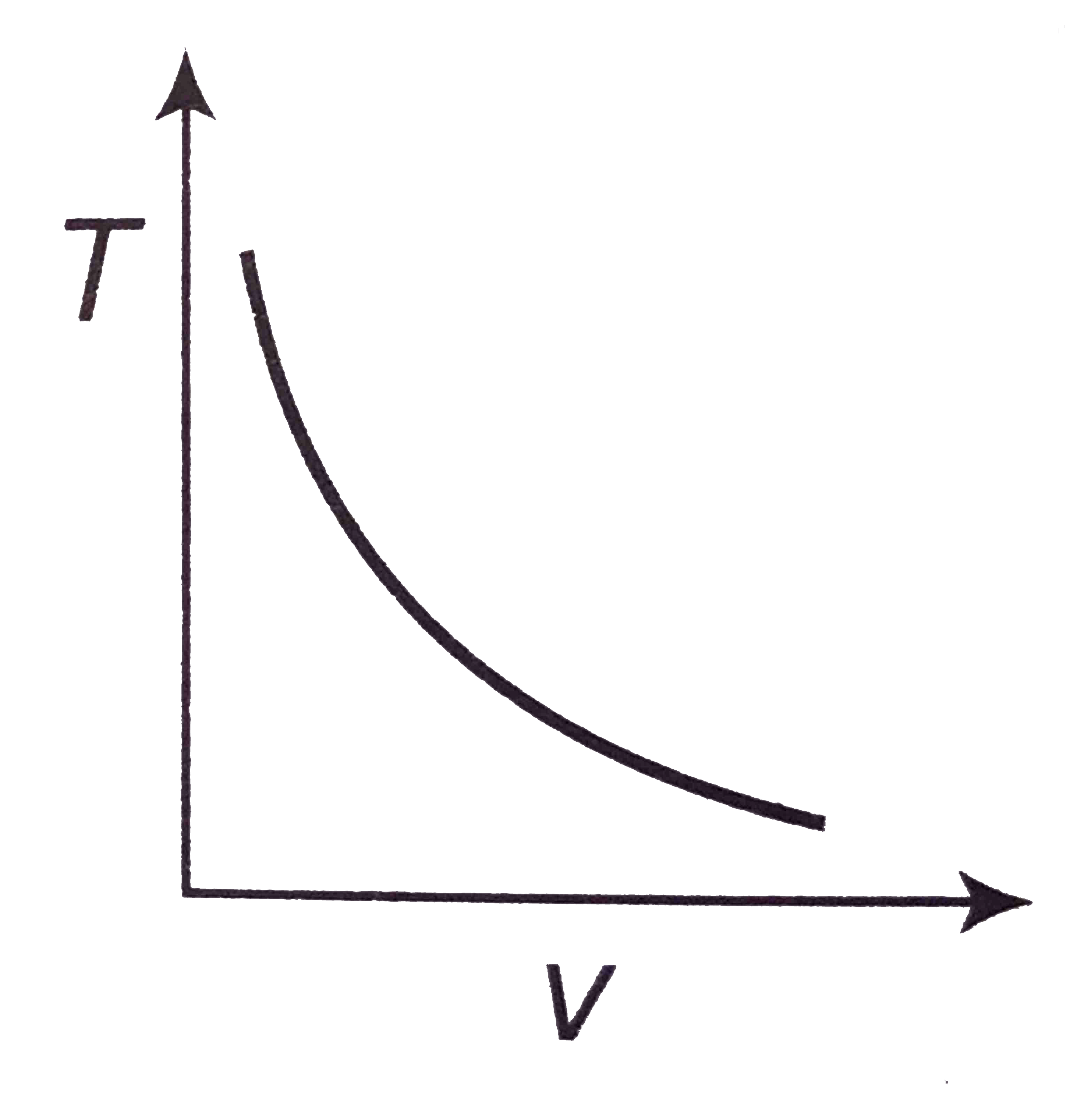 C
C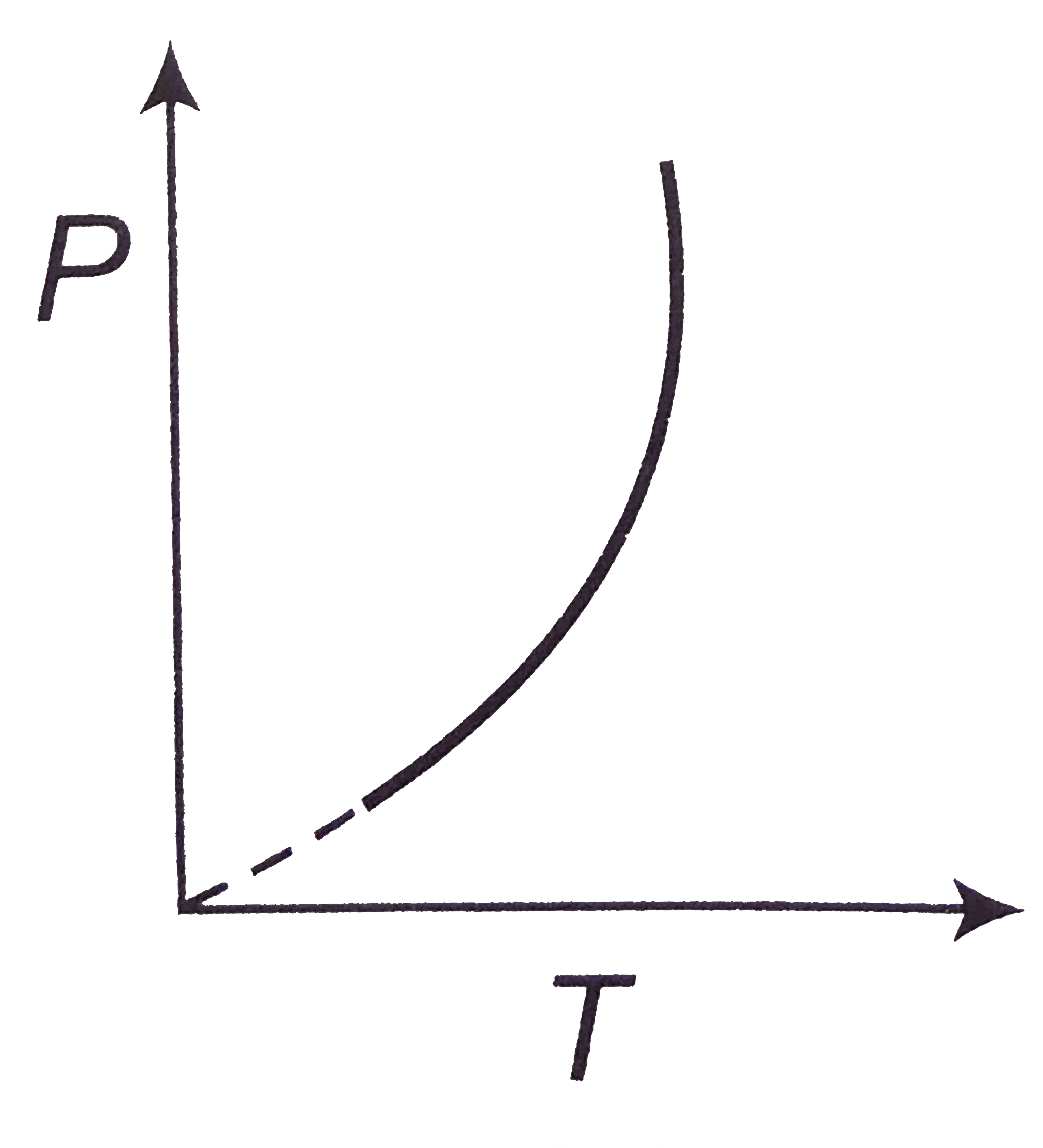 DAll option are correct
DAll option are correctWhich of the following is false regarding reversible adiabatic expansion of an ideal gas ?
APlot of T us V is a straight line with slope equal to γBPlot of ln T us ln V is a striaght line with slope equal to γCPlot of ln T us ln V is a straight line with slope equal to −γDPlot of ln T us ln V is a straight line with slope equal to 1−γ
Which of the following expression is/are correct for a adiabatic process?
View SolutionWhich of the following is correct for adiabatic expansion ideal gas?
View SolutionWhich of the following is (are) correct an adiabatic process?
View SolutionFor the adiabatic expansion of an ideal gas:
View SolutionWhich of the following is zero during reversible adiabatic expansion of the gas
View Solution