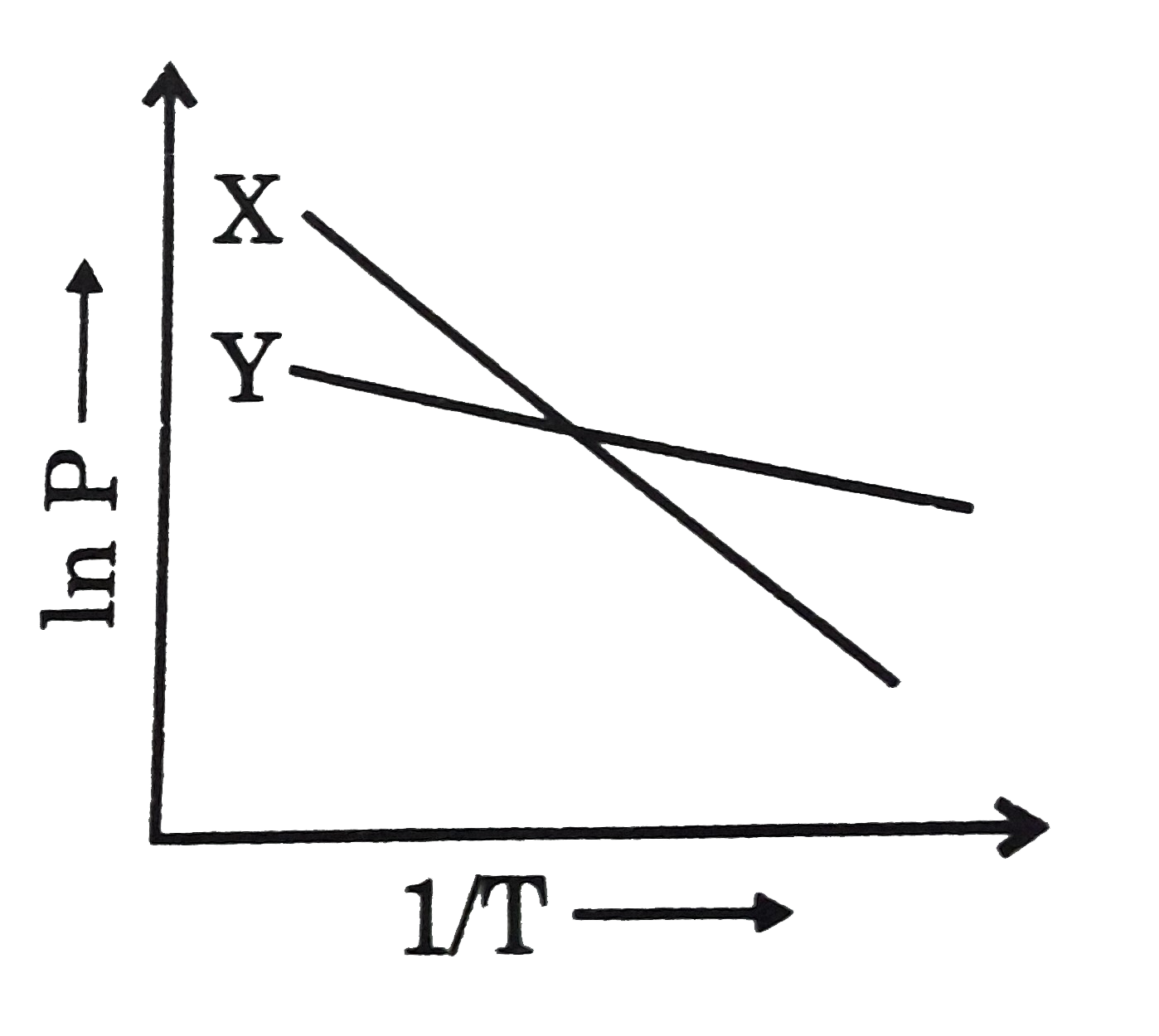
What can be concluded about the values of ΔH and ΔS from this graph ?
A
ΔH>0,ΔS>0
B
ΔH>0,ΔS<0
C
ΔH<0,ΔS>0
D
ΔH<0,ΔS<0
Video Solution
Text Solution
The correct Answer is:A
|
Answer
Step by step video, text & image solution for What can be concluded about the values of DeltaH and DeltaS from this graph ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
What can be concluded about the substance represented by this phase diagram ?
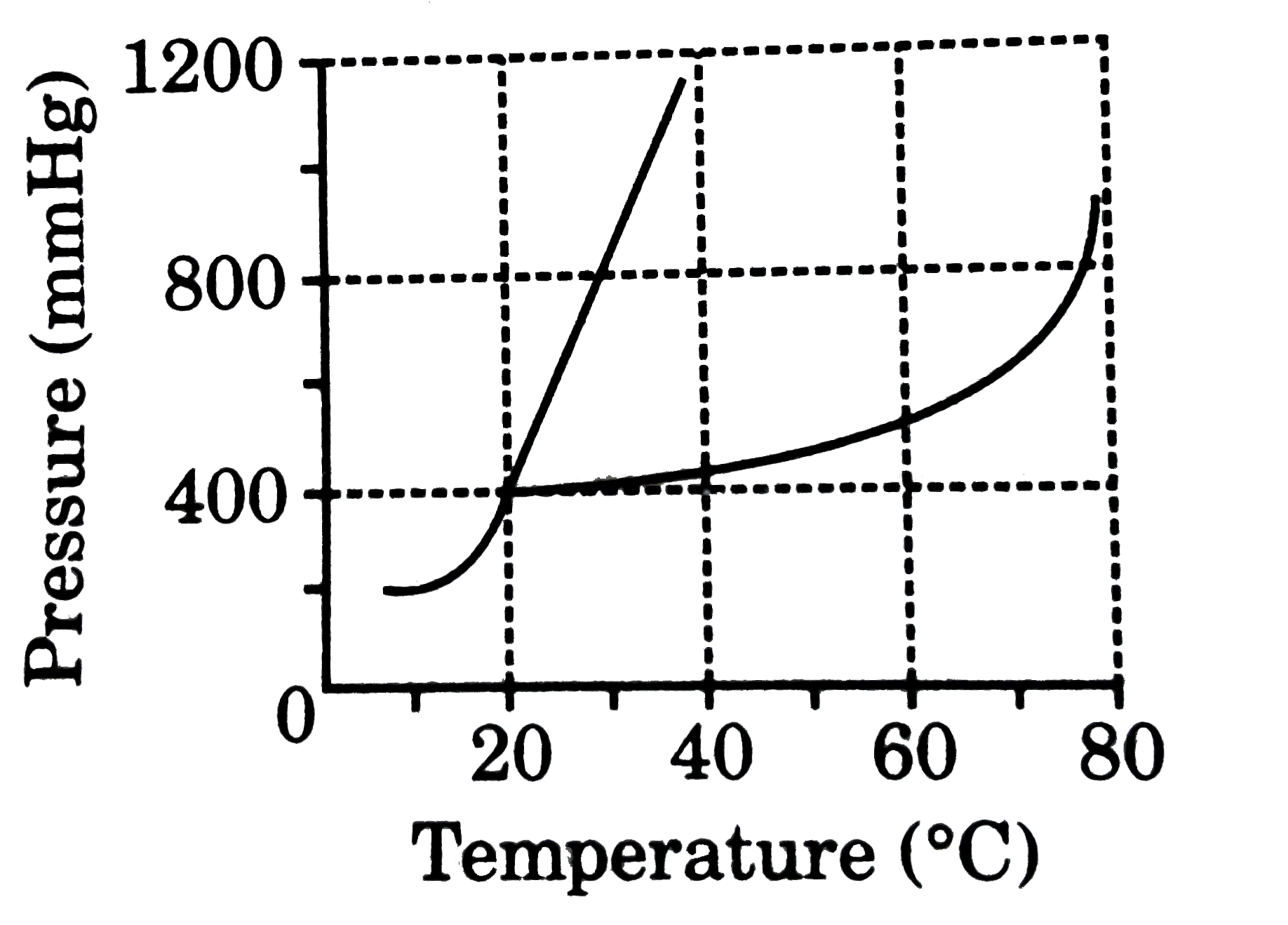 AThe normal boiling point of the liquid is 80∘CBThe solid is more dense than the liquidCThe solid sublimes at temperatures above 20∘C.DThe vapour can be converted to liquid by compressing it at temperatures below 20∘C
AThe normal boiling point of the liquid is 80∘CBThe solid is more dense than the liquidCThe solid sublimes at temperatures above 20∘C.DThe vapour can be converted to liquid by compressing it at temperatures below 20∘CWhen solid NH4NO3 is dissolved in water at 25∘C, the temperature of the solution decreases. What is true about the signs of ΔHandΔS for this process?
AΔHΔS−+BΔHΔS−−CΔHΔS++DΔHΔS+−When solid NH4NO3 is dissolved in water at 25∘C, the temperature of the solution decreases. What is true about the signs of ΔHandΔS for this process?
AΔHΔS−+BΔHΔS−−CΔHΔS++DΔHΔS+−
In evaporation of water, ΔHandΔS are
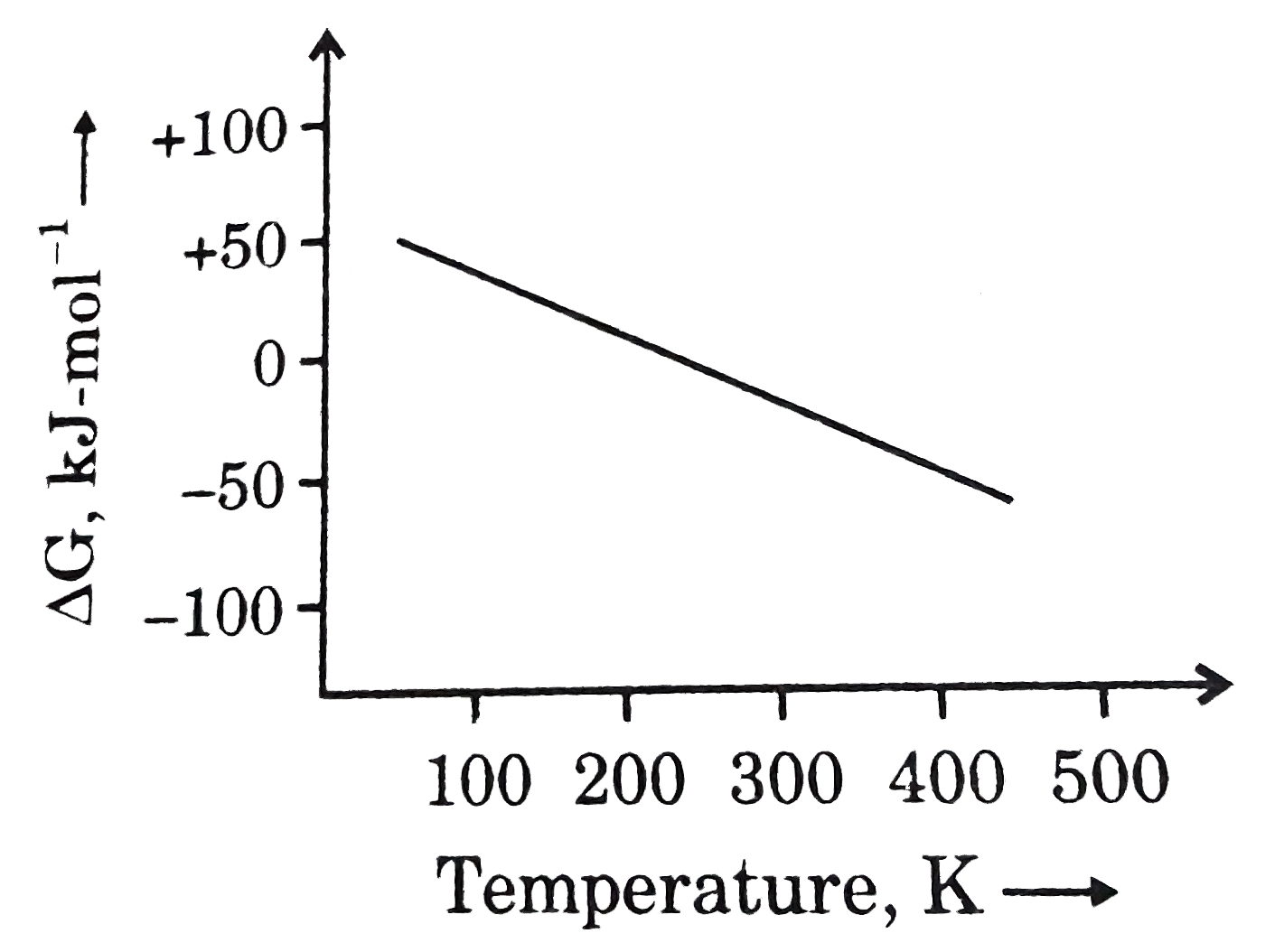
View SolutionWhat can be said from graph below?
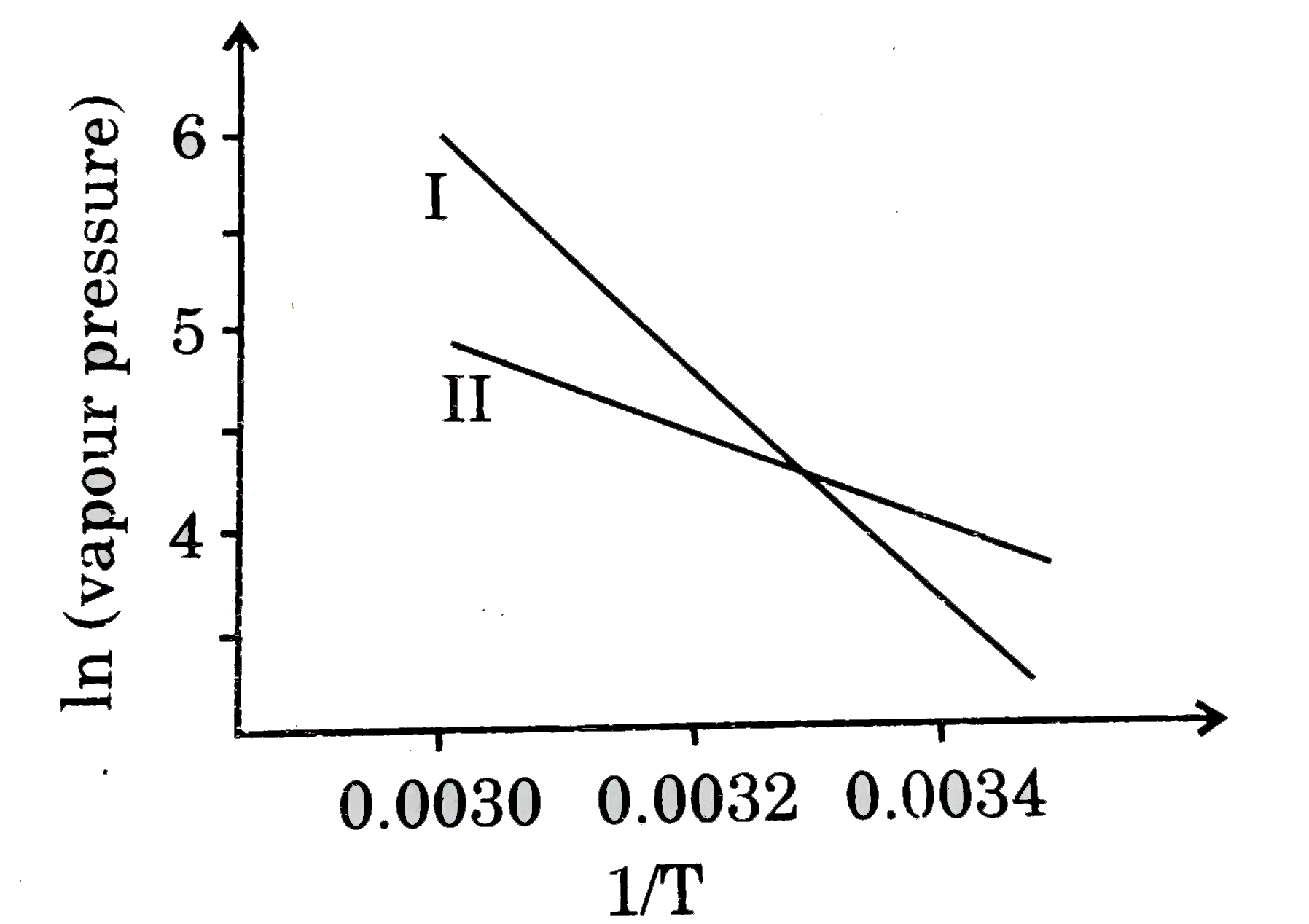
View SolutionIn the graph the natural log of the vapour pressures of two substances are plotted vs 1/T. what can be concluded about the relative the 1/T enthalpies of vaporization (ΔHvap) of these substances?
View SolutionWhat are the sign of ΔHandΔS for a reaction that is spontaneous only at low temperatures?
View SolutionFor which change ΔH≠ΔE :-
View Solution