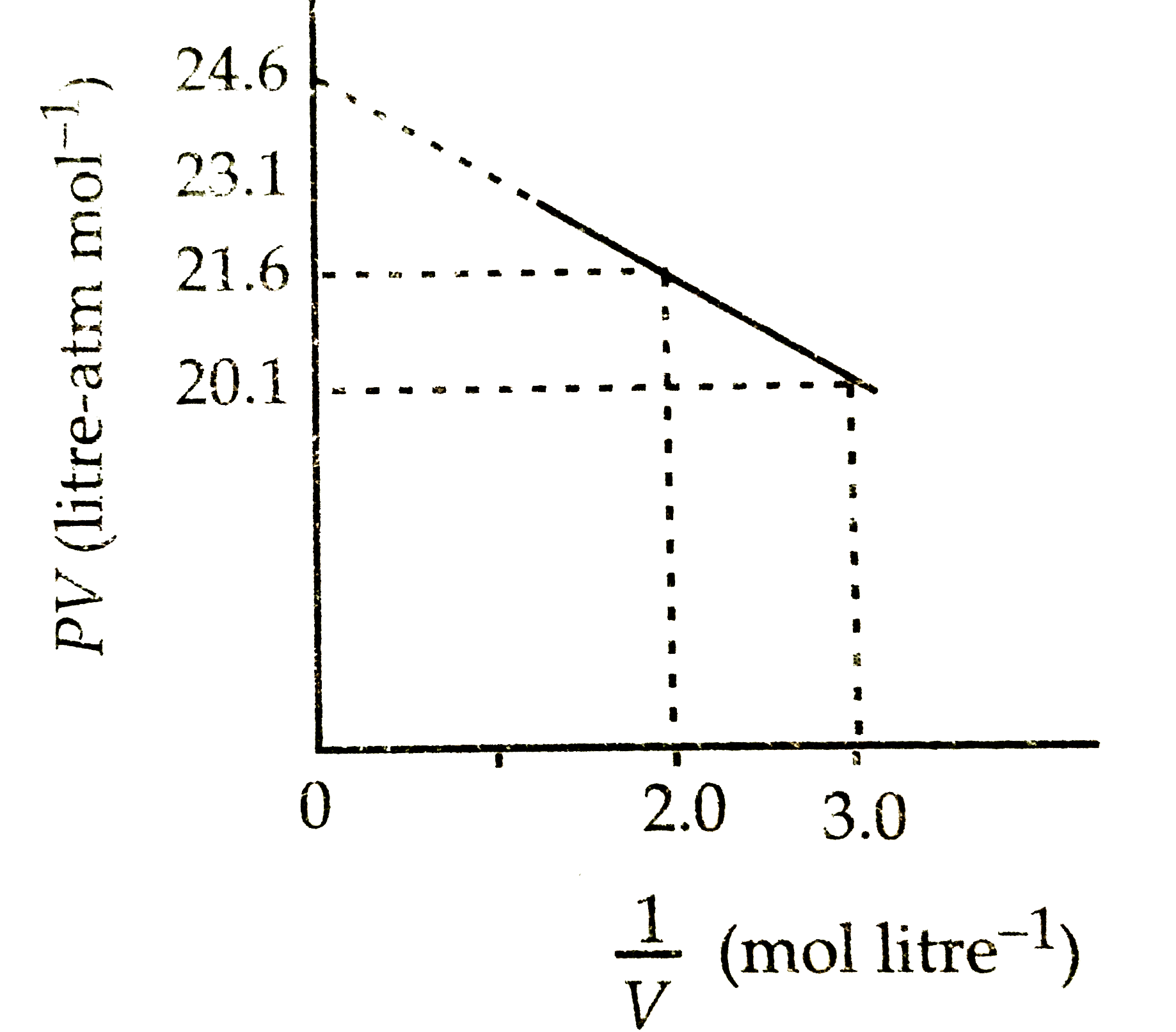
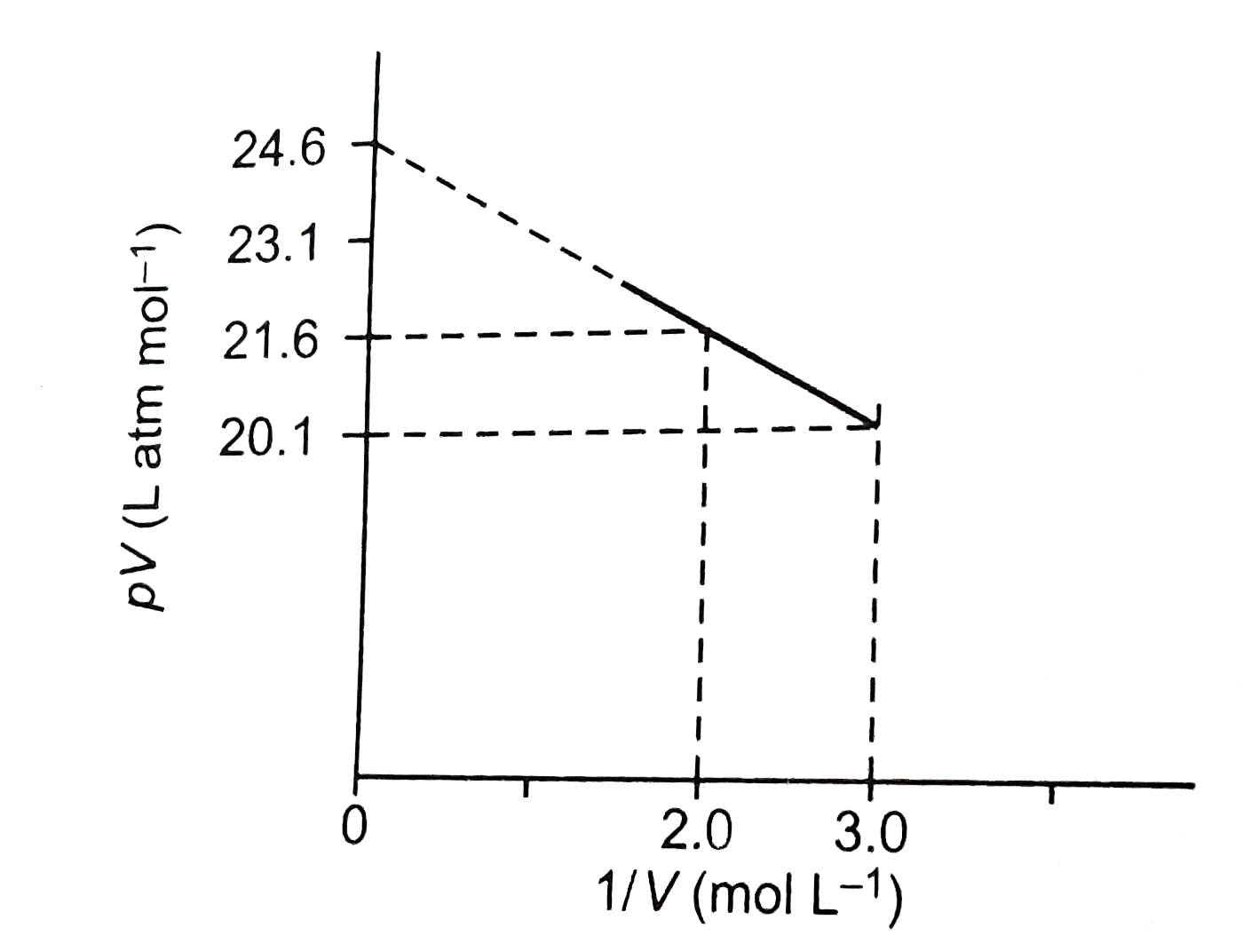
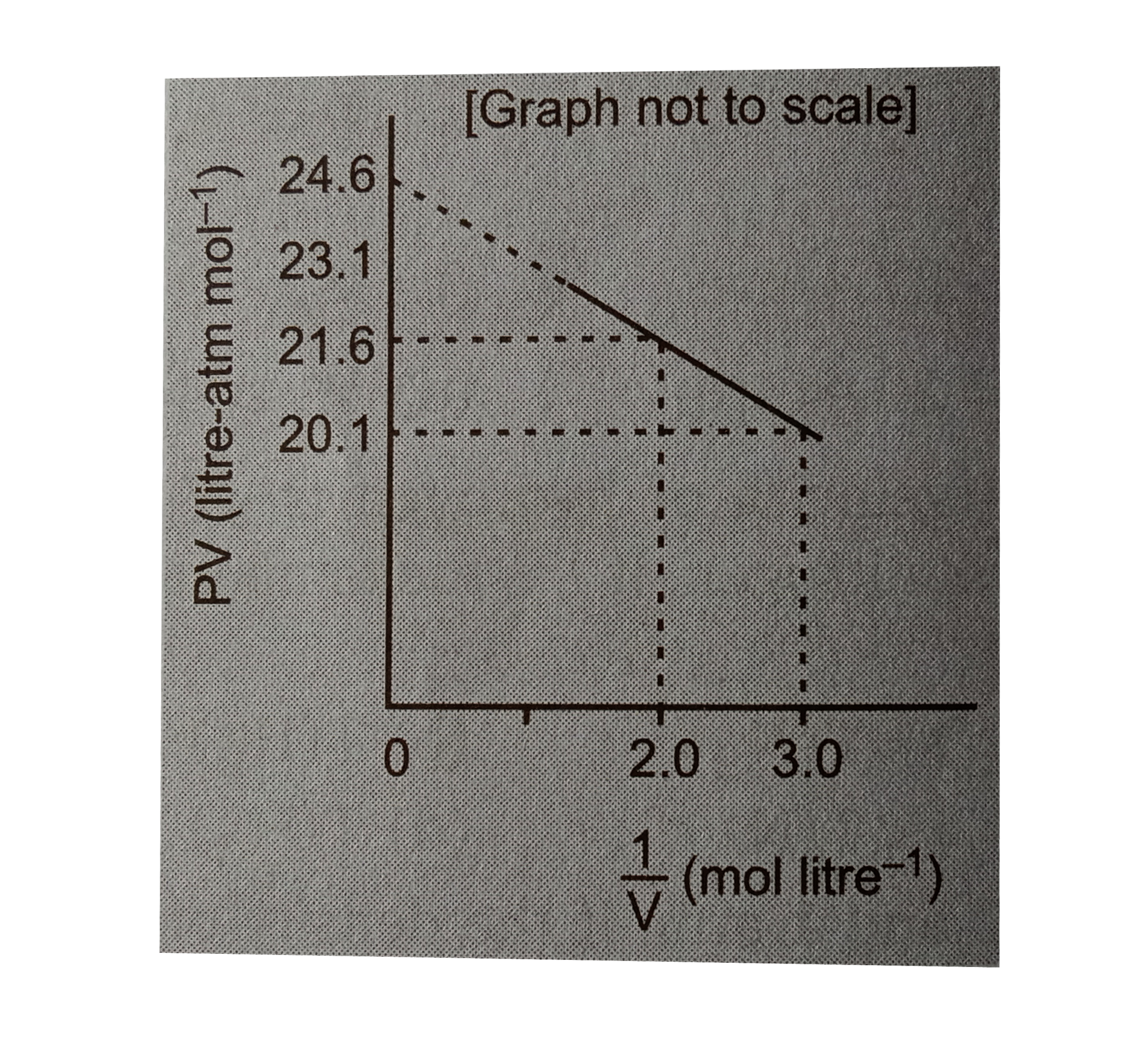
For one mole of a van der Waal's gas when b=0 and T=300K, the PV us 1/V plot is shown ahead. The value of the van der Waal's constant a(atm. litre2mol−2) is:
A
1.0
B
4.5
C
1.5
D
3.0
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
For one mole of a van der Waals' gas when b=0 and T=300K, the pVvs1/V plot is shown below. The value of the vander Waals' constant a(atm Lmol−2)
 A1B4.5C1.5D3
A1B4.5C1.5D3For one mole of a van der Waals gas when b=0 and T=30K the PVvs1/V plot is shown below The value of the van Waals constant a (atm litre2mol−2) is
 .A1.0B4.5C1.5D3.0
.A1.0B4.5C1.5D3.0The units of the van der Waal's constant 'b' are
AL mol−1BjoulesCL mol−1Dmol L−1
The units of the van der Waal's constant 'b' are
View SolutionThe units of the van der Waal's constant 'b' are
View SolutionThe value of van der Waals constant 'a' is makimum for
View SolutionThe value of van der Waals constant a is the maximum for
View SolutionThe van der Waal's gas constant, a is given by
View Solution