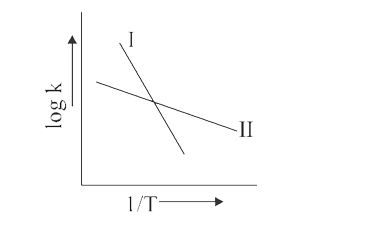
The Arrhenius relationship of two different reactions is shown below. Which reaction is faster at a lower temperature and which is more sensitive to changes of temperature ?
B faster, A more sensitive
B in both cases
A in both cases
A is faser, B more sensitive
The correct Answer is:A
Step by step video, text & image solution for The Arrhenius relationship of two different reactions is shown below. Which reaction is faster at a lower temperature and which is more sensitive to changes of temperature ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Rate of which reaction increase with temperature:
AAnyBExothermic of the reactantCEndothermic reactionsDNone of the aboveIn which case is a reaction possible at any temperature ?
AΔH<0,ΔS>0BΔH<0,ΔS<0CΔH>0,ΔS>0Din none of the casesIn which case is a reaction impossible at any temperature ?
AΔH>0,ΔS>0BΔH>0,ΔS<0CΔH<0,ΔS<0Din all cases
Which reaction is affected to a much lesser extent by temperature?
View SolutionRate of which reactions increases with temperature:
View SolutionRate of which reactions increases with temperature:
View SolutionWhich of the reaction is expectd to be spontaneous at low temperatures and non-spontaneous at high temperatures:
View SolutionIn which of the following cases, the reaction is spontaneous at all temperatures
View Solution