Rate of reaction A→B depends only on A and can be represented by below curve then:
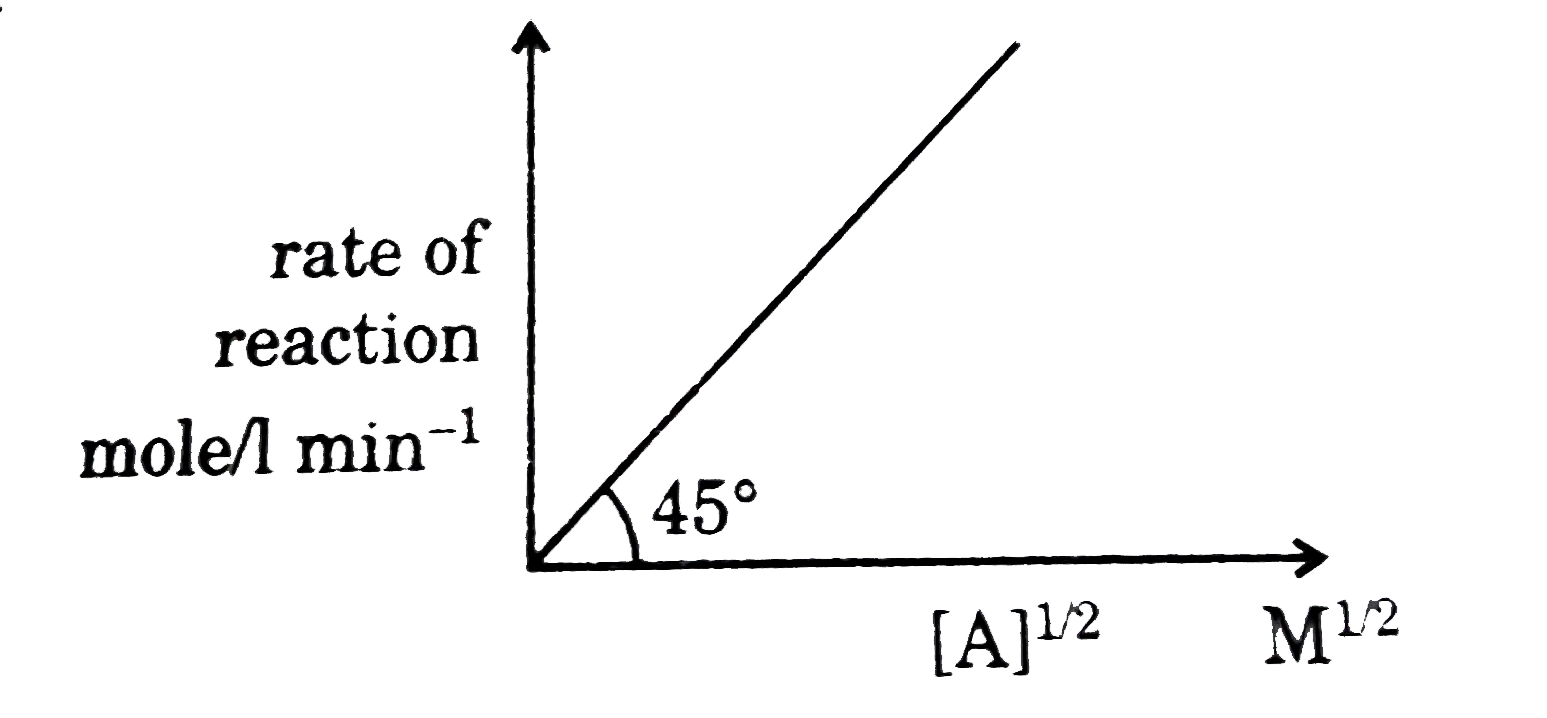
A
molecularity of reaction must be one
B
reaction will be an elementary reaction
C
value of rate constant for reaction will be 1M−1/2sec−1
D
reaction will definitely be a complex reaction
Video Solution
Text Solution
The correct Answer is:D
|
Answer
Step by step video, text & image solution for Rate of reaction A rarr B depends only on A and can be represented by below curve then: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The rate of a reaction depends on the
Atemperature of the reactionBcatalystCconcentration of the reactantsDall of theseThe rate of a reaction depends upon the
AVolumeBForceCPressureDConcentration of reactantThe rate of a chemical reaction depends on
AAtomic MassBEquivalent MassCMolecular MassDActive Mass
The rate constant of a reaction depends on
View SolutionFor the reaction 2A+3B→4C the rate of reaction may be represented as
View SolutionThe rate constant of a reaction depends on
View Solution
The rate of the reaction depends on the concentration of
View SolutionThe rate constant of a reaction depends on
View Solution

