The conversion of vinyl allyl ether to pent-4-enol follows first-order kinetics. The following plot is obtained for such a reaction. The rate constant for the reaction is:
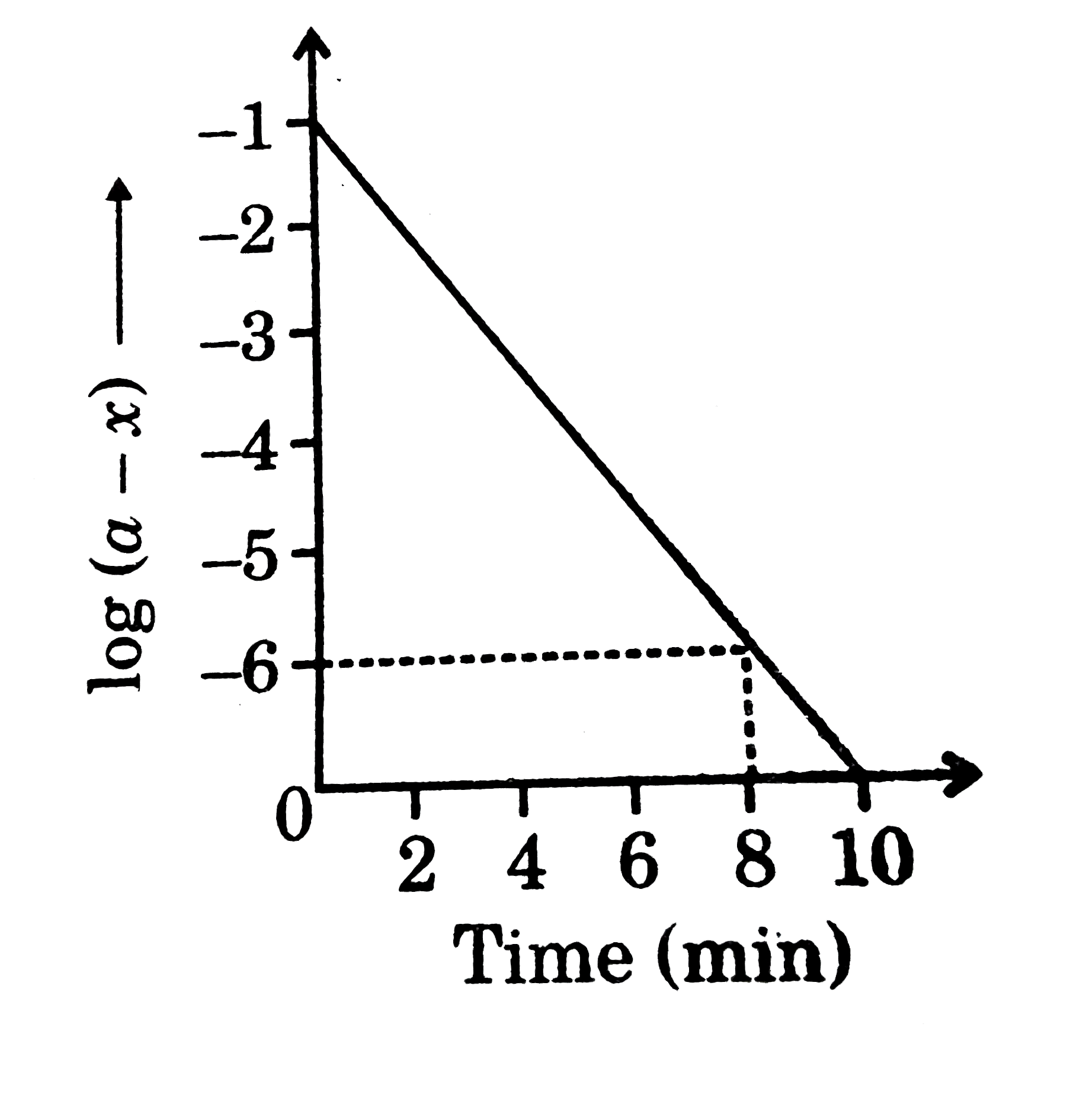
A
4.6×10−2s−1
B
1.2×10−2s−1
C
2.3×10−2s−1
D
8.4×10−2s−1
Video Solution
Text Solution
The correct Answer is:C
|
Answer
Step by step video, text & image solution for The conversion of vinyl allyl ether to pent-4-enol follows first-order kinetics. The following plot is obtained for such a reaction. The rate constant for the reaction is: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The converison of vinyl allyl ether to pent-4-enol follows first order kinetics. The following plot is obtained for such a reaction:
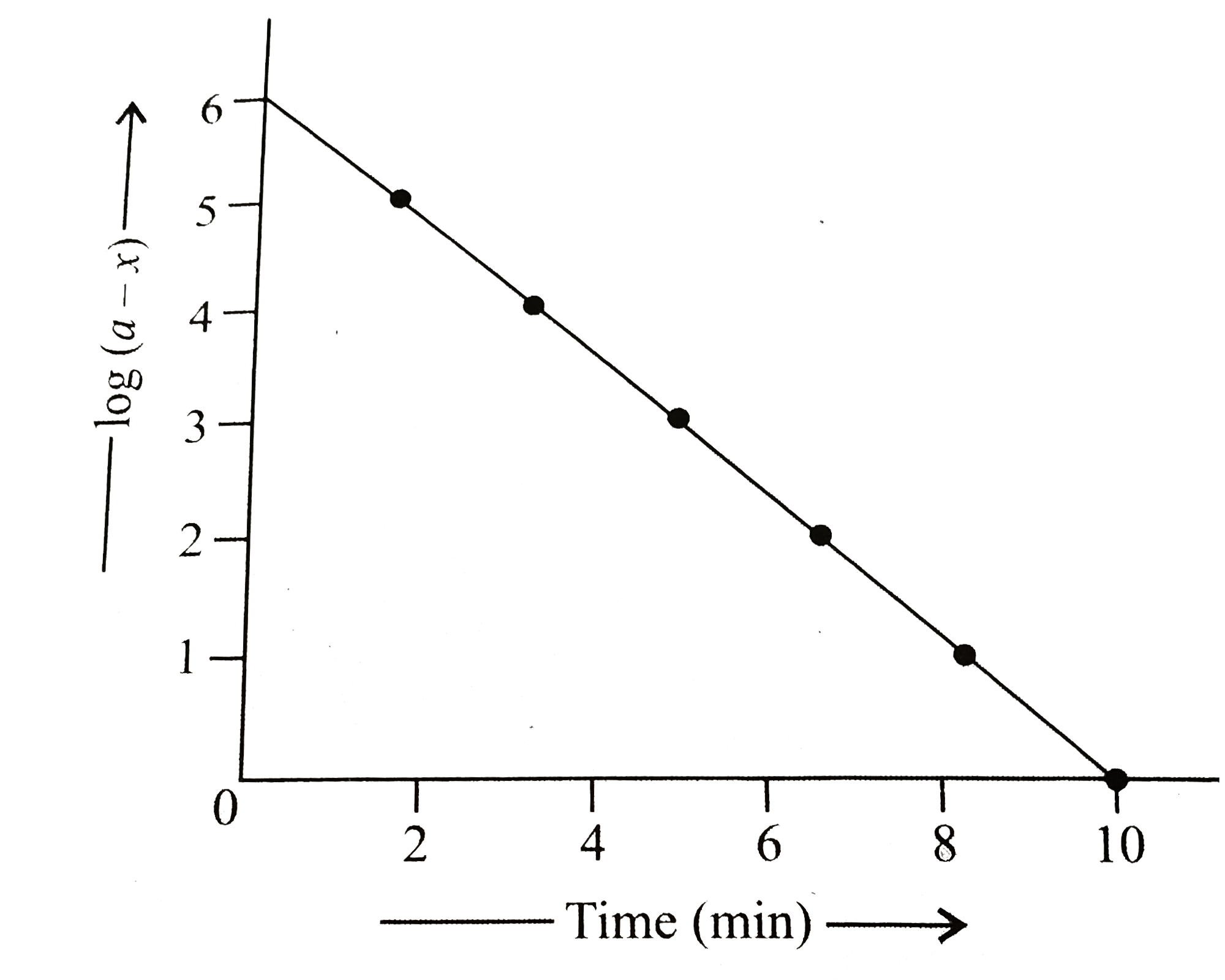
The rate constant for the reaction isA4.6×10−2s−1B1.2×10−2s−1C2.3×10−2s−1D8.4×10−2s−1The unit of the rate constant for first order reaction is
Amol / litB(mol / lit ) time −1Ctime −1D(mol/lit) timeThe unit of rate constant for first order reaction is
Amin−2BsCs−1Dmin
Units for the rate constant of first order reaction is
View SolutionThe rate constant of a first order reaction is given by
View SolutionThe unit of rate constant for a first order reaction
View SolutionThe rate constant 'K' for pseudo first order reaction is
View SolutionThe integrated rate equation for rate constant of a first order reaction is:
View Solution

