Titration curves for 0.1 M solutions of three weak acids HA1,HA2andHA3 with ionization constant K1,K2,K3 respectively are plotted as shown in the following figure. Which of the following is/are true.?
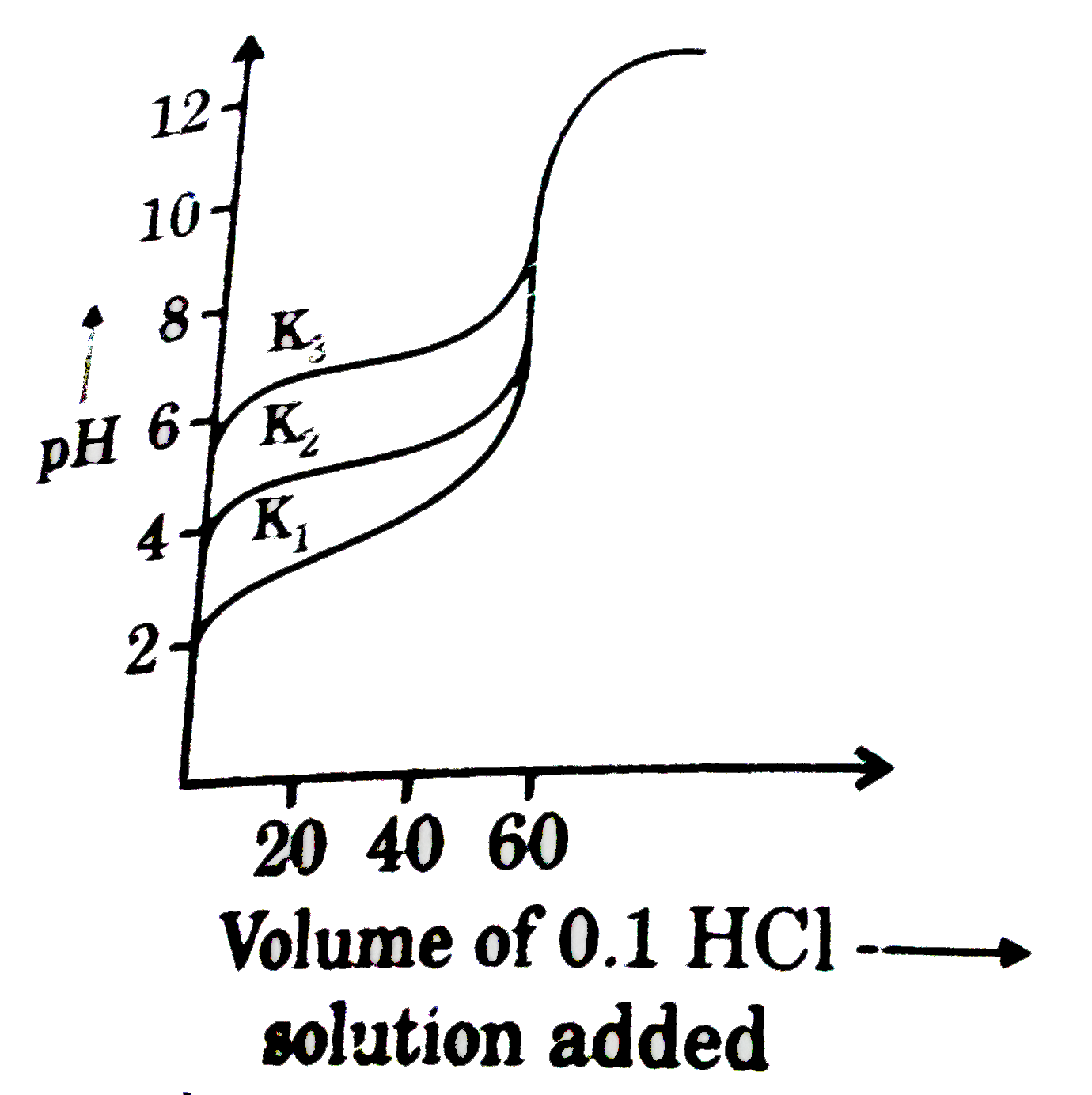
A
K2=(K1+K3)2
B
K1<K3
C
K1>K2
D
K2>K3
Text Solution
The correct Answer is:C, D
|
Similar Questions
Knowledge Check
Titration curves for 0.10M solution of three weak acids HX,HY and HZ with ionization constants H1,K2 and K3 respectively are plotted as shown in the figure below:
 AK2=K1+K22BK1<K3CK1>K2DK2>K3
AK2=K1+K22BK1<K3CK1>K2DK2>K3If the concentration of the weak monoprotic acid HA is C mmol L−1 and its ionization constant is Ka, then
AC+H=C/2BC+H=√CCC+H=√KaCDC+H=C/CaThe pH of a 0.1 molar solution of the acid HQ is 3. The value of the ionisation constant, Ka of the acid is
A3×10−1B1×10−3C1×10−5D1×10−7
Ka1,Ka2andKa3 are the three dissociation constants of H3PO4. Which of the following is a correct order
View SolutionWhich of the following aromatic acids has lowest Ka ?
View SolutionFor a 0.1 M aqueous solution of a weak acis, HA (Ka=10−9), the pH is approximately equal to
View SolutionIf the dissociation constants of two weak acids HA1 and HA2 are K1 and K2, then the relative strengths of HA1 and HA2 are given by
View SolutionFor a diprotic acid, which of the following is true for 1st and 2nd ionization constants (Ka1 and Ka2)
View Solution

