A weak acid (or base) is titrated against a strong base (or acid), volume V of strong base (or acid) is plotted against pH of the solution (as shown in fig).The weak electrolyte (i.e., acid or base) could be:
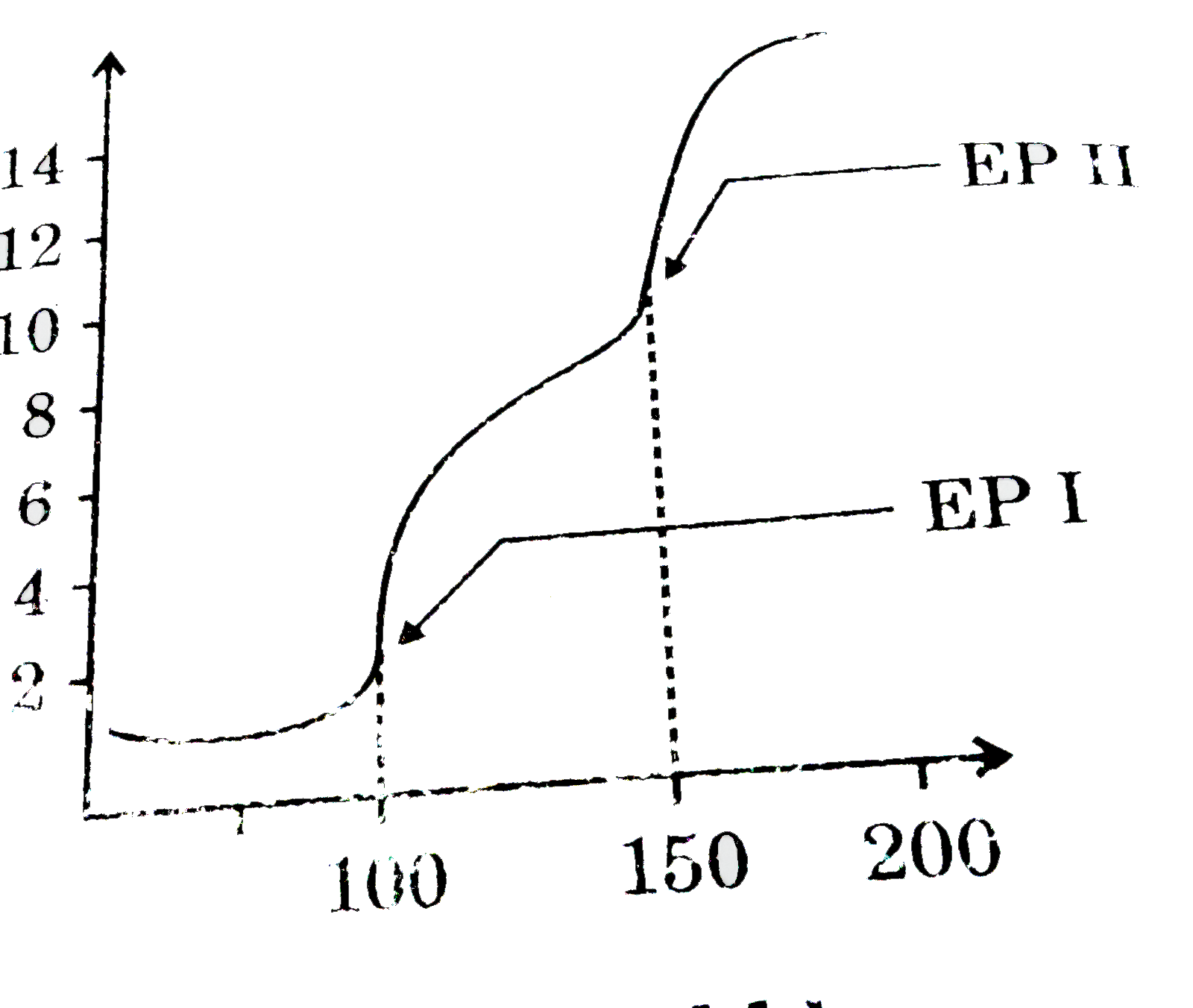
A
Na2CO3
B
Na2C2O4
C
H2C2O4
D
CH4(COOH)2
Text Solution
The correct Answer is:C, D
|
Similar Questions
Knowledge Check
A solution of a substances is titrated against a strong base ( or acid ) ,volume V of strong base (or acid) is plotted against pH of the solution (as shown in figure) .The substances could be :
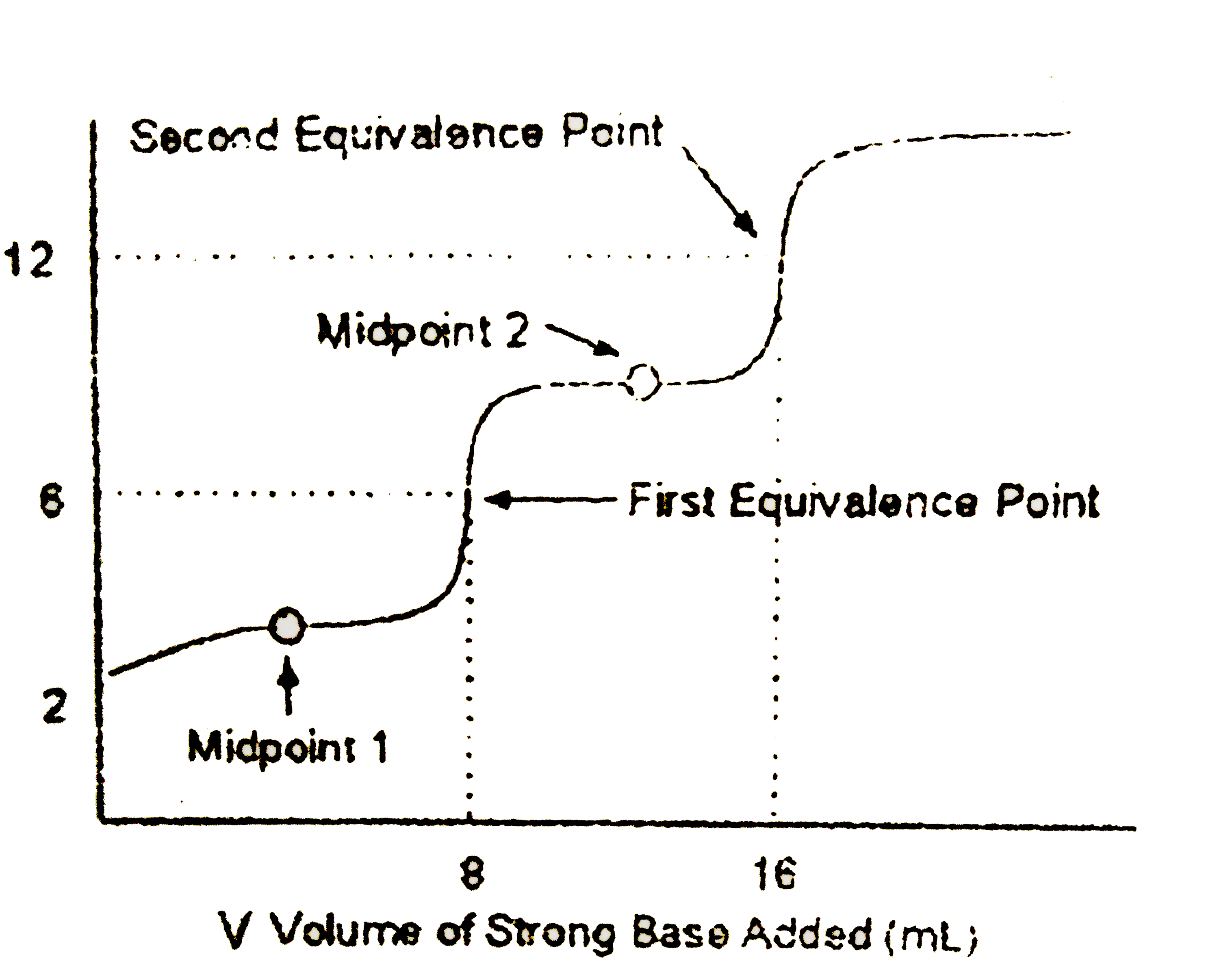 ANa2CO3BEthylene diamineCH2C2O2DCH2(COOH)2
ANa2CO3BEthylene diamineCH2C2O2DCH2(COOH)2pH of a salt of a strong base with weak acid
ApH=12pKw+12pKa+12logCBpH=12pKw−12pKa−12logCCpH=12pKw+12pKa−12logCDNone of theseWhen a salt of strong base and weak acid is hydrolysed, the resulting solution has :
ApH=7BpH=0CpH<7DpH>7
A weak base (BOH) with Kb=10−5 is titrated with a strong acid (HCl), At 3/4 th of the equivalence point, pH of the solution is:
View SolutionAqueous solution of salt of strong base and weak acid
View SolutionA strong acid is titrated with weak base. At equivalence point, pH will be :
View SolutionA salt derived from a strong base and weak acid will give a salt that is
View SolutionA weak monobasic acid is half neutralized by a strong base. If the Ph of the solution is 5.4 its pKa is
View Solution



