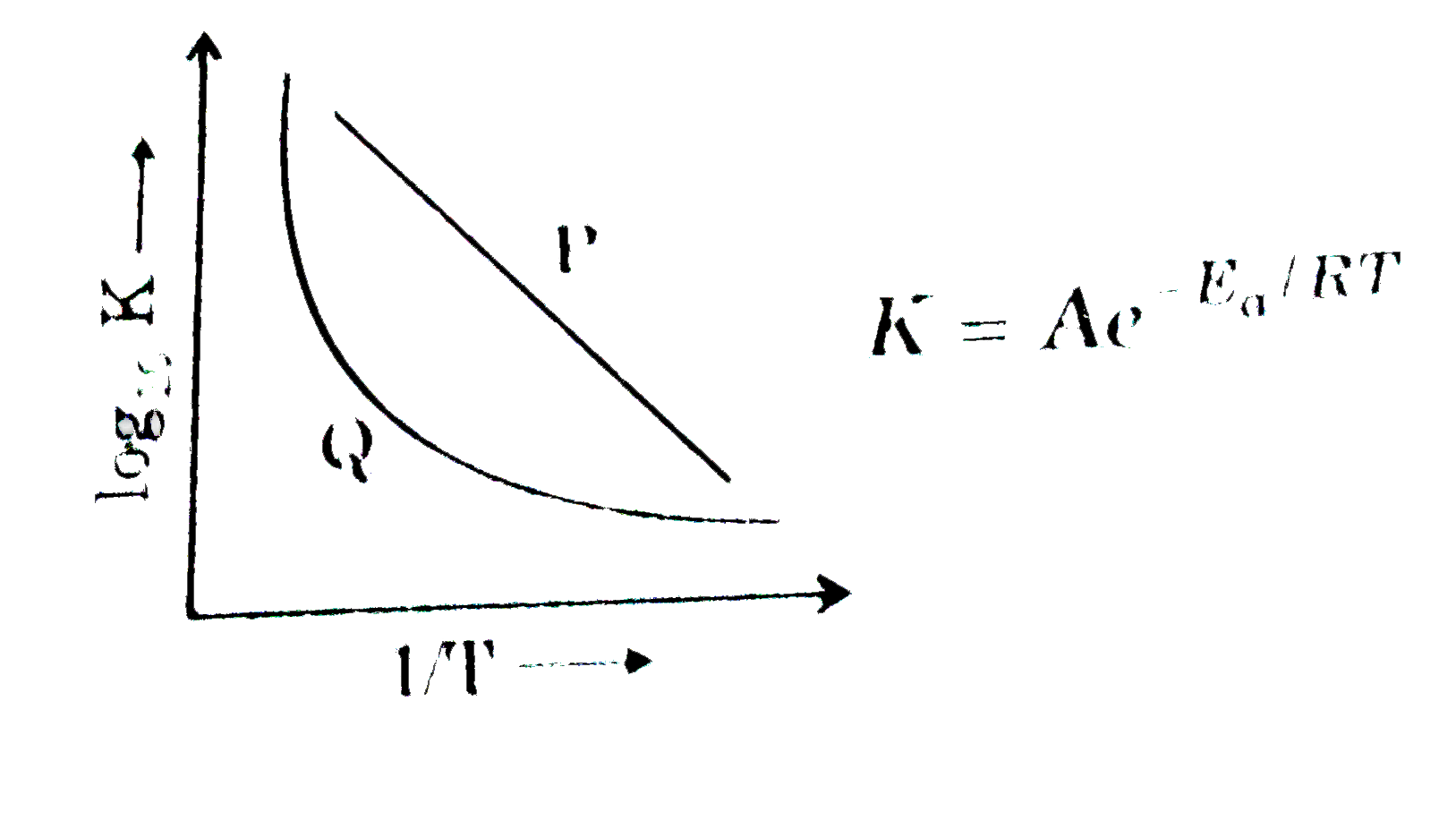
Which of the following statement are true regarding the log K vs 1/T. Plot shown in the given diagram?
A
Plot P shows that the energy of activation is independent of temperature
B
Plot Q describes the behaviour of temperature dependence of energy of activation
C
Arrhenius behaviour is described by P
D
The slope of curve P gives the value −EaR
Text Solution
The correct Answer is:A, B, C, D
|
Similar Questions
Knowledge Check
Which of the following statements regarding the given compounds are true ?
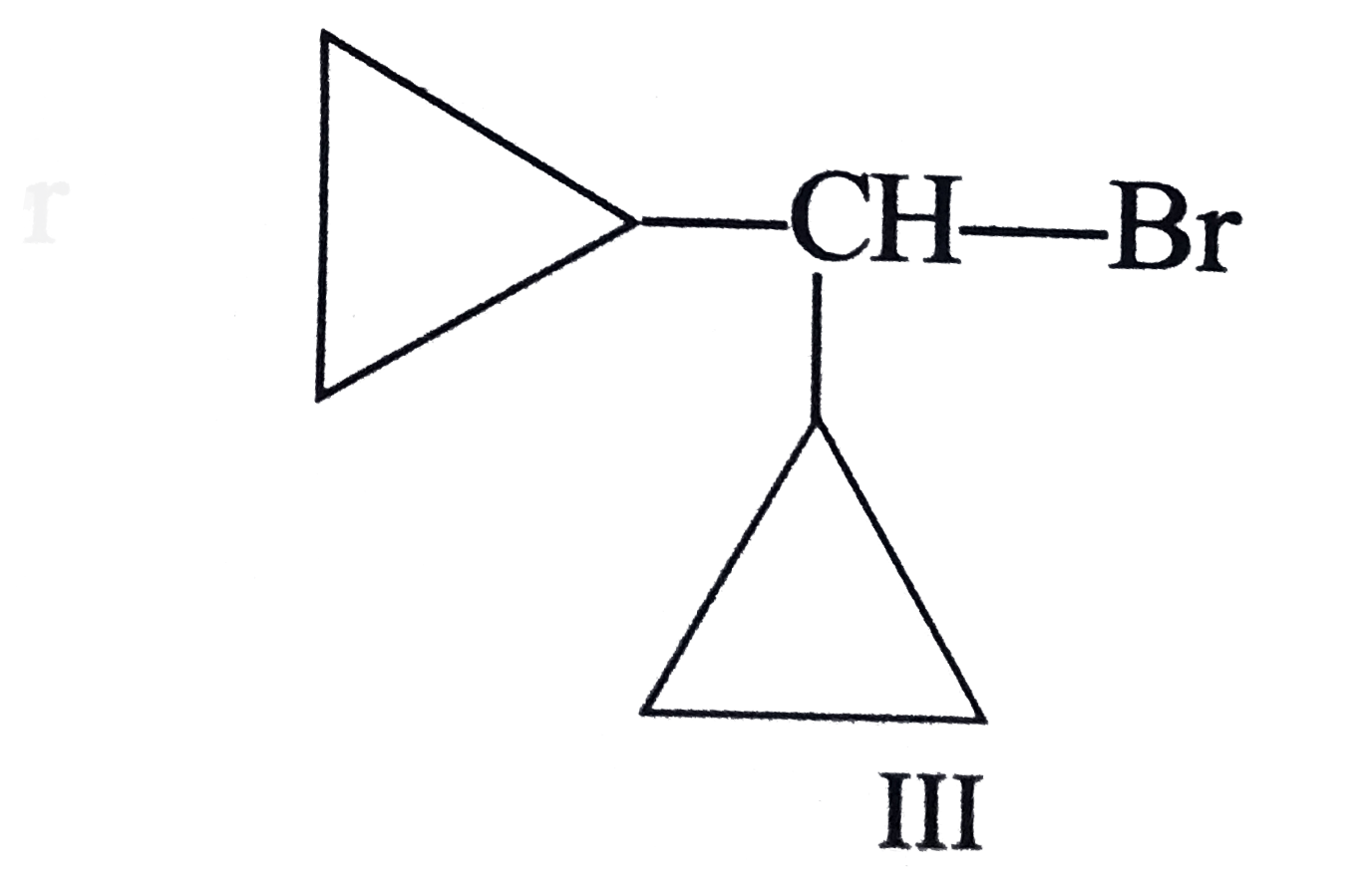 AReactivity for SN1 is III>II>IBReactivity for SN1 is I>III>IICReactivity for SN2 is II>III>IDReactivity for SN2 is II>I>III
AReactivity for SN1 is III>II>IBReactivity for SN1 is I>III>IICReactivity for SN2 is II>III>IDReactivity for SN2 is II>I>IIIIn a plot of log k vs 1/T, the slope is
A−Ea/2.303BEa/2.303RCEa/2.303D−Ea/2.303RIn a plot of log k vs 1/T, the slope is
A−Ea/2.303BEa/2.303RCEa/2.303D−Ea/2.303R
Which of the plots of ln K vs (1T) is/are correct?
View SolutionIn a plot of log k vs 1/T, the slope is
View SolutionSelect the incorrect statements about the plots of ln K vs 1/T
View SolutionWhich of the plots of ln K vs (1T) is/are correct?
View SolutionIn a plot of log.k vs 1/T, the slope'is :
View Solution

