If critical temperature for the real gas is 500 K then the value of :
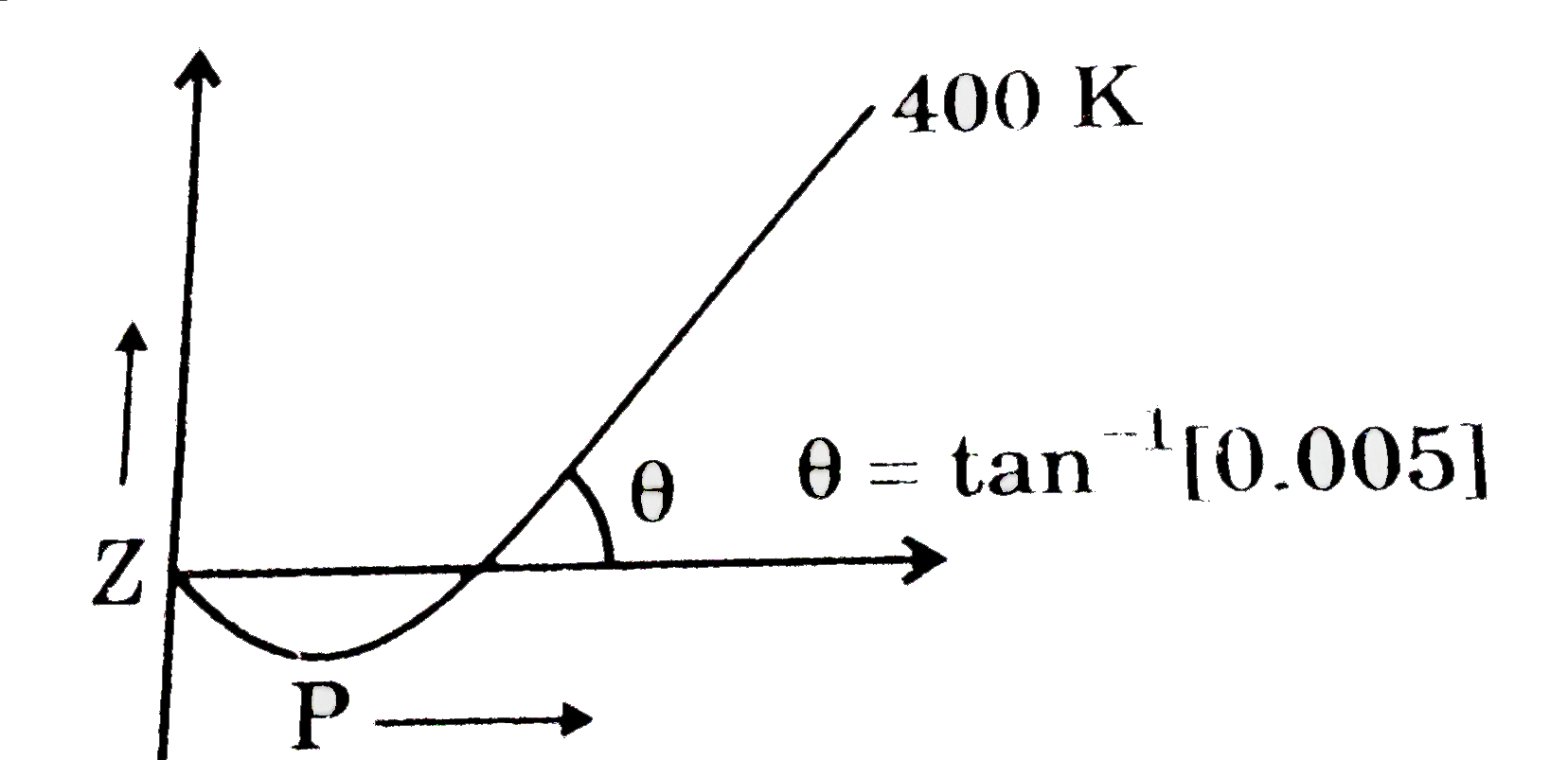
A
Boyle's temperature is 1687.5K
B
van der Waals' constant a for the gas is 21.6 atm-L2/mol2
C
Gas will show ideal behaviour at high temperature and low pressure
D
At Boyle's temperature behaviour of gas is always ideal
Video Solution
Text Solution
The correct Answer is:A, C
|
Answer
Step by step video, text & image solution for If critical temperature for the real gas is 500 K then the value of : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
At critical temperature:
Aliquid passes into gaseous state imperceptibly and continouslyBgas passes into liquid state imperceptibly and continouslyCsurface separating two phases disappearsDall of the aboveAt the critical temperature
Aliquid and vapour exist in equilibriumBvapour state does not exist at allCthe meniscus between liquid and vapour disappearsDthe vapour condense into solidAt the critical temperature , the substance exists as a
ALiqued and vapour exist in equilibriumBvapour state does not exist at allCthe meniscus between liquid and vapour disappearsDthe vapour condense into solid
Critical temperature of the gas is the temperature
View SolutionThe critical temperature of the gas is the temperature
View SolutionThe critical pressure for real gases is given by
View SolutionThe critical temperature of a gas is that temperature:
View SolutionThe higher the critical temperature of the gas
View Solution
