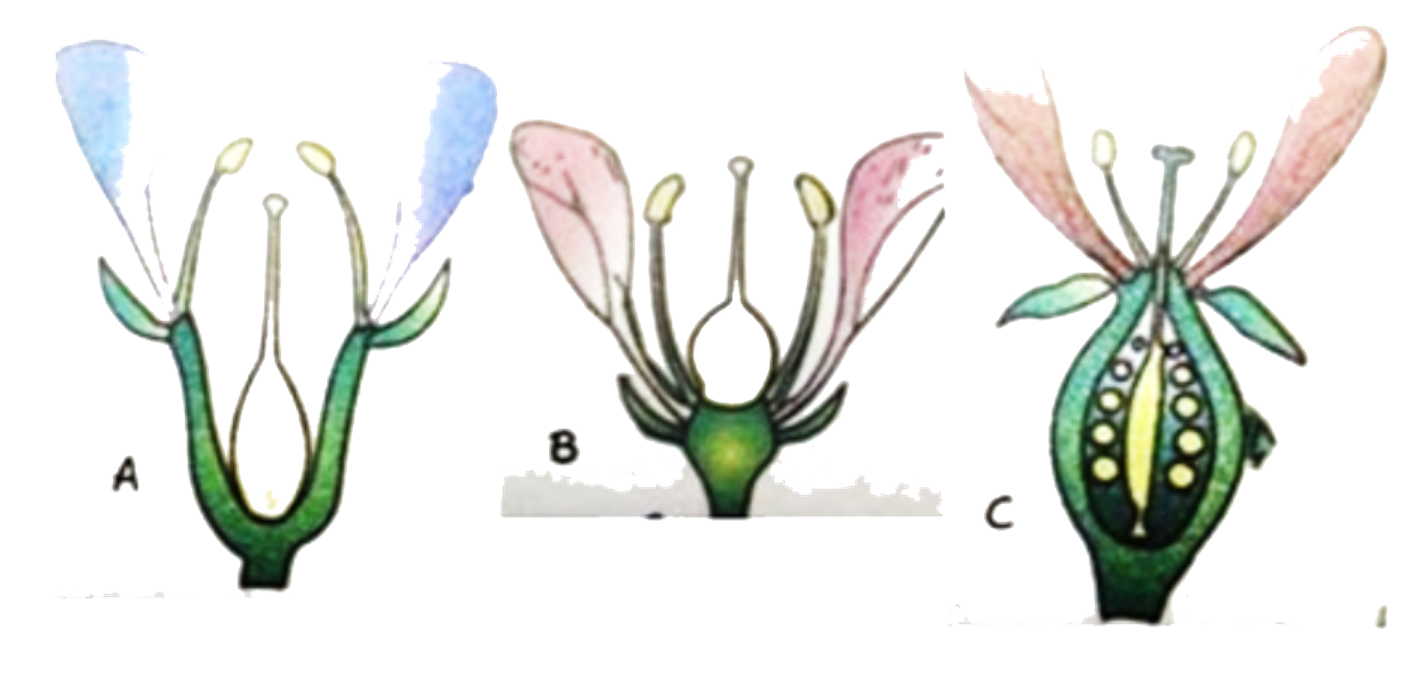
For an ideal gas, following observation is made as per Maxwell-Boltzmann distrbution. Choose the correct options.
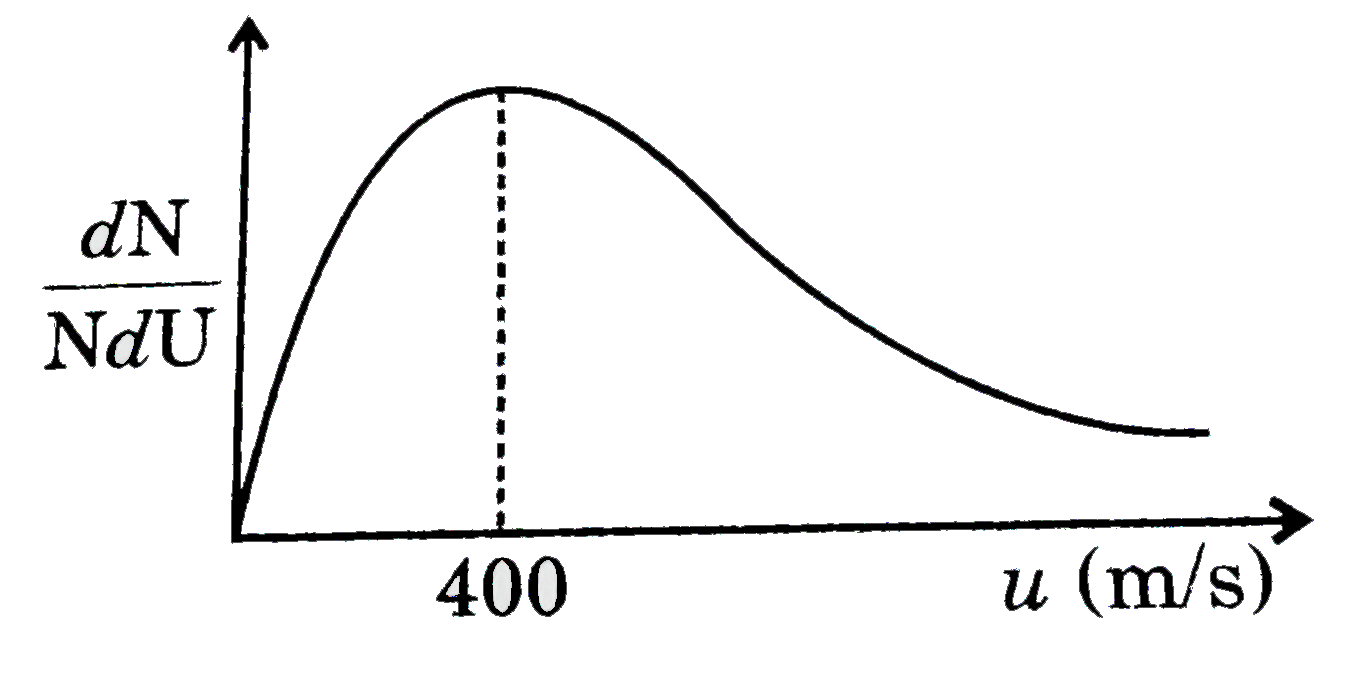
μrms=√1.5×400m/s
μavg=√4π×400m/s
Fraction of molecules moving between 400 to 401 m/s are more than fraction of molecules moving between 452 to 453 m/s
Fraction of molecules moving between 400 to 401 m//s are equal with fraction of molecules moving between 452 to 453 m/s
The correct Answer is:A, B, C
Step by step video, text & image solution for For an ideal gas, following observation is made as per Maxwell-Boltzmann distrbution. Choose the correct options. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- GRAPHICAL INTERPRETATIONBOOK - GRB PUBLICATIONCHAPTER - GRAPHICAL INTERPRETATIONEXERCISE - Comprehension 1
1 Video
- GRAPHICAL INTERPRETATIONBOOK - GRB PUBLICATIONCHAPTER - GRAPHICAL INTERPRETATIONEXERCISE - Comprehension
49 Videos
- GRAPHICAL INTERPRETATIONBOOK - GRB PUBLICATIONCHAPTER - GRAPHICAL INTERPRETATIONEXERCISE - Electrochemistry
9 Videos
- F-BLOCK ELEMENTSBOOK - GRB PUBLICATIONCHAPTER - F-BLOCK ELEMENTSEXERCISE - Subjective type
7 Videos
- HYDROCARBON (ALIPHATIC)BOOK - GRB PUBLICATIONCHAPTER - HYDROCARBON (ALIPHATIC)EXERCISE - Previous years jee questions
28 Videos
Similar Questions
Knowledge Check
For ideal gas, observation as per Maxwell distribution
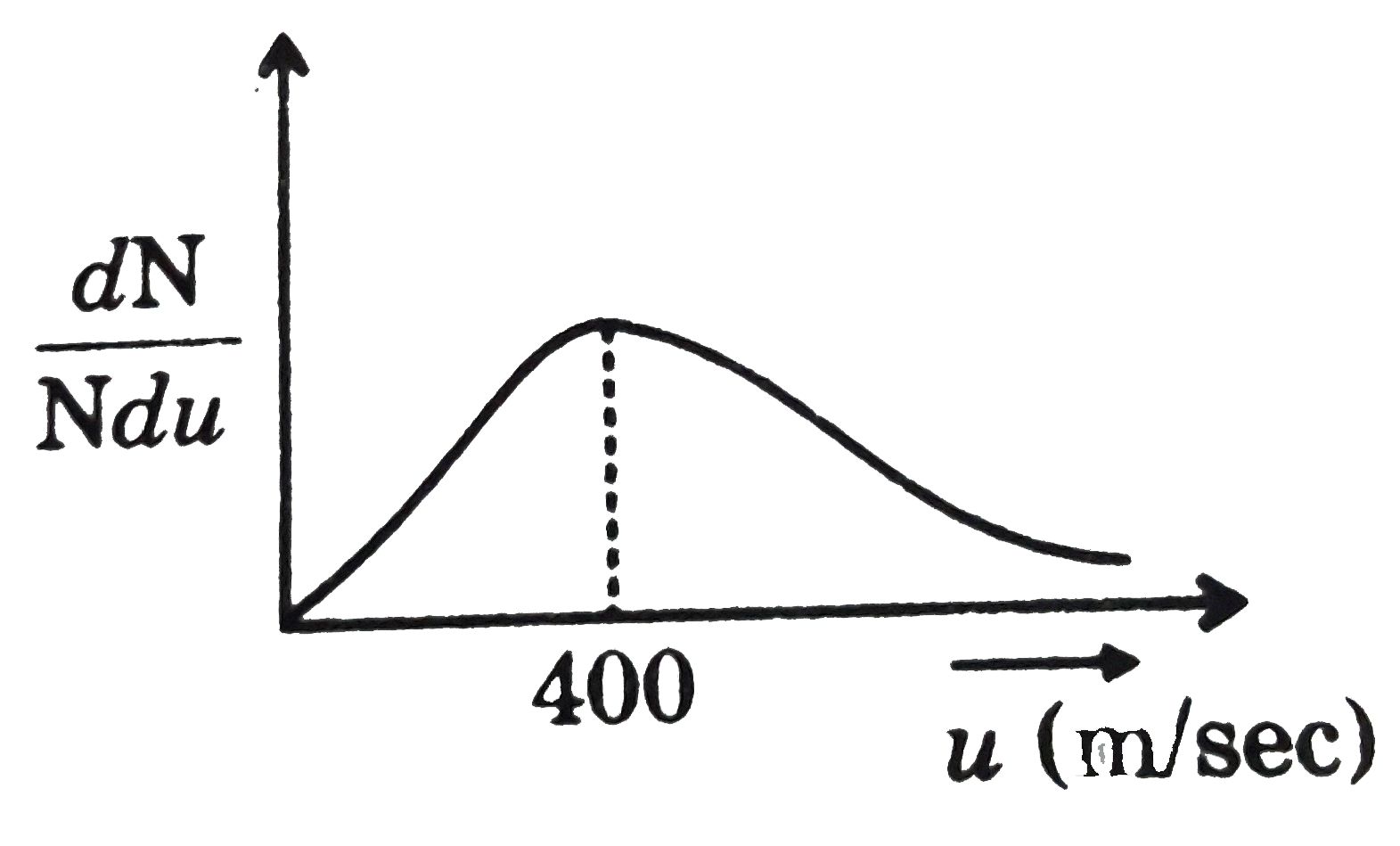 AUrms=√1.5×100m/sBFraction of molecules moving between 400 to 401 m/sec are equal with fraction of molecules moving between 452 to 453 m/sec.CUrms=√20×400m/sDUavg=1.5Umps
AUrms=√1.5×100m/sBFraction of molecules moving between 400 to 401 m/sec are equal with fraction of molecules moving between 452 to 453 m/sec.CUrms=√20×400m/sDUavg=1.5UmpsFor an ideal solution, the correct option is:
AΔmixS=0 at constant T and PBΔmixV≠0 at constant T and PCΔmixH=0 at constant T and PDΔmixG=0 at constant T and PFor an ideal solution, the correct option is
AΔmixG=0 at constant T and PBΔmixS=0 at constant T and PCΔmixV≠0 at constant T and PDΔmixH=0 at constant T and P
Choose the correct options from the following:
View SolutionFor an ideal solution, the correct option is:
View SolutionMatch the following columns and choose the correct option.

View SolutionMatch the following and choose the correct options

View SolutionMatch the following and choose the correct options

View Solution