Which of the following are correct ?
When 2 moles of an ideal gas (CV=3R2) is heated from 300 K to 600 K at constant pressure, change in entropy of gas is 5R ln 2
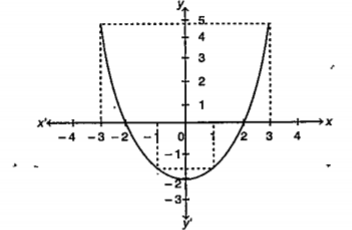
An ideal gas is undergoing in process as shown in figure. Work done for process 1-2 and 1-3 will be equal .

Entropy change when an ideal gas exapnds isothermally is positive
Entropy change is positive for an adiabatic free expansion of an ideal gas
The correct Answer is:A, B, C, D
Similar Questions
Which at the following pairs are correct?
View SolutionMultiple correct postmodern Which of the following statements is correct?
View SolutionWhich of the following statements is correct:
View SolutionWhich of the following statements "is"//"are" correct?
View SolutionWhich of the following relationships is not correct ?
View SolutionChoose the correct option.
Which is following relations is correct?
View Solutionसही उत्तर चुनें-
निम्नलिखित में कौन सही है ?
View Solutionwhich of the following is correct for the following graph?
View Solution

