Which of the following are correct ?
A
For van der Waal's gas, the pressure at which gas may behave ideally is aVmb in low pressure range.
B
For chemical reaction at state of chemical equilibrium (ΔG)TP must be zero
C
The expansion of van der Waal's gas in isothermal process occurs with increase in internal energy.
D
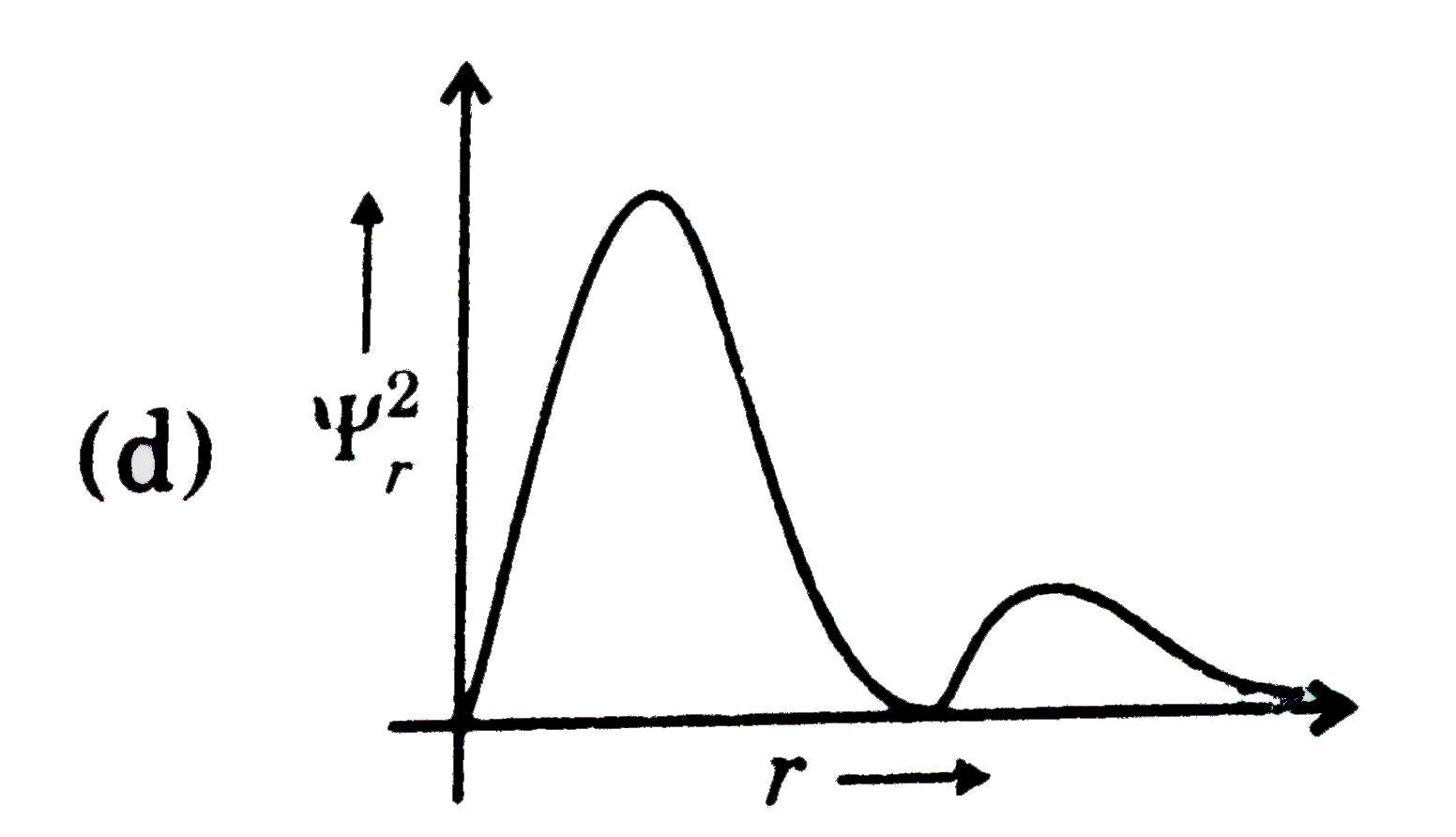
The orbital represented by the above graph will have on radial node and Ψangular depends on θ.
Text Solution
The correct Answer is:A, B, C, D
|
Similar Questions
Which of the following satements is not correct?
View Solutionনীচের কোন্ অনুক্রমটি সঠিক ?
View SolutionChoose the correct option.
Which is following relations is correct?
View Solutionसही उत्तर चुनें-
निम्नलिखित में कौन सही है ?
View Solutionwhich of the following is correct for the following graph?
View SolutionWhich of the following statements is/are correct?
View Solution

