If an element can exist in several oxidation states, it is convenient to display the reduction potentials corresponding to the various half reactions in diagrammatic from, known as Latimer diagram. The Latimer diagram for chlorine in acid solution is
Cl−4+1.20V−−−−→ClO−3+1.60V−−−−→ClO−2+1.60V−−−−→
ClO−+1.67V−−−−→Cl2+1.36V−−−−→Cl−
In basic solution is :
ClO−4+0.37V−−−−→ClO−3+0.30V−−−−→ClO−2+0.68−−−→
ClO−+0.42V−−−−→Cl2+1.36−−−→Cl−. The standard potentials for two nonadjacent species can also be calculated by using the concept that ΔG∘ is an additive property but using potential is not an assitive property and ΔG∘=−nFx0, If a given oxidation state is a the next higher oxidation state disproportionation can occur. The reverse of relative stabilities of the oxidation state can also be understood by drawing a graph of ΔG∘/F against oxidation state, known as Frost diagram, Choosing the stability of zero oxidation state arbitrery as zero. The most stable oxidation state of a apecies lies lowest in the digram Disproportionation is spontaneous if the species lies above a straight line joining its two product species.
Which of the following statement is correct according to above question ?
A+1 undergoes disproportionation into A and A2+
A2+ undergoes disproportionation in A and A3+
A undergoes comproportionation into A+1 and A−1
All of the above
The correct Answer is:A
Topper's Solved these Questions
- GRAPHICAL INTERPRETATIONBook:GRB PUBLICATIONChapter:GRAPHICAL INTERPRETATIONExercise:Comprehension 2
Explore 1 Video
- GRAPHICAL INTERPRETATIONBook:GRB PUBLICATIONChapter:GRAPHICAL INTERPRETATIONExercise:Comprehension 3
Explore 1 Video
- GRAPHICAL INTERPRETATIONBook:GRB PUBLICATIONChapter:GRAPHICAL INTERPRETATIONExercise:Comprehension 1
Explore 1 Video
- F-BLOCK ELEMENTSBook:GRB PUBLICATIONChapter:F-BLOCK ELEMENTSExercise:Subjective type
Explore 7 Videos
- HYDROCARBON (ALIPHATIC)Book:GRB PUBLICATIONChapter:HYDROCARBON (ALIPHATIC)Exercise:Previous years jee questions
Explore 28 Videos
Similar Questions
If an element can exist in several oxidation states, it is convernient to display the reduction potentials correspondingg to the various half reactions in diagrammatic form, know as latimer diagram the latimer diagram for chlorine in acid solutio is
CIO−4+1.20V−−−−→CiO−3+1.18V−−−−→HClO2+1.60V−−−−→HClO1.67V−−→Cl21.36V−−→Cl− in basic solution.
ClO−40.37V−−→ClO−30.30V−−→ClO−20.68V−−→ClO−0.42V−−→Cl21.36V−−→Cl− The standard potentials for two nonadjacent species can also be calculateed by using the concept that △G∘ as an additive property but potential is not an additive property and △G∘=−nFx0. if a given oxidation stateis a stronger oxidising agent than the next higher oxidation state, disproportionation can occur. The reverse of disproportionation is called comproportionation. The relative stabilities of the oxidation state can also be understood by drawing a graph of △G∘/F against oxidation state, known as frost diagram, choosing the stability of zero oxidation state arbitrarily as zero. The most stable oxidation state of a species lies lowest in the diagram, disproportionation is spontaneous if the species lies above a straight line joining its two product species.
Q. Which of the following statement is correct?
View SolutionIf an element can exist in several oxidation states, it is convernient to display the reduction potentials correspondingg to the various half reactions in diagrammatic form, know as latimer diagram the latimer diagram for chlorine in acid solutio is
CIO−4+1.20V−−−−→CiO−3+1.18V−−−−→HClO2+1.60V−−−−→HClO1.67V−−→Cl21.36V−−→Cl− in basic solution.
ClO−40.37V−−→ClO−30.30V−−→ClO−20.68V−−→ClO−0.42V−−→Cl21.36V−−→Cl− The standard potentials for two nonadjacent species can also be calculateed by using the concept that △G∘ as an additive property but potential is not an additive property and △G∘=−nFx0. if a given oxidation stateis a stronger oxidising agent than the next higher oxidation state, disproportionation can occur. The reverse of disproportionation is called comproportionation. The relative stabilities of the oxidation state can also be understood by drawing a graph of △G∘/F against oxidation state, known as frost diagram, choosing the stability of zero oxidation state arbitrarily as zero. The most stable oxidation state of a species lies lowest in the diagram, disproportionation is spontaneous if the species lies above a straight line joining its two product species.
Q. What is the potential couple ClO−Cl− at pH=14?
View Solution
Knowledge Check
If an element can exist in several oxidation states, it is convenient to display the reduction potentials corresponding to the various half reactions in diagrammatic from, known as Latimer diagram. The Latimer diagram for chlorine in acid solution is
Cl−4+1.20V−−−−→ClO−3+1.60V−−−−→ClO−2+1.60V−−−−→
ClO−+1.67V−−−−→Cl2+1.36V−−−−→Cl−
In basic solution is :
ClO−4+0.37V−−−−→ClO−3+0.30V−−−−→ClO−2+0.68−−−→
ClO−+0.42V−−−−→Cl2+1.36−−−→Cl−. The standard potentials for two nonadjacent species can also be calculated by using the concept that ΔG∘ is an additive property but using potential is not an assitive property and ΔG∘=−nFx0, If a given oxidation state is a the next higher oxidation state disproportionation can occur. The reverse of relative stabilities of the oxidation state can also be understood by drawing a graph of ΔG∘/F against oxidation state, known as Frost diagram, Choosing the stability of zero oxidation state arbitrery as zero. The most stable oxidation state of a apecies lies lowest in the digram Disproportionation is spontaneous if the species lies above a straight line joining its two product species.
What is the potential of couple ClO−Cl− at pH = 14 14?A1.78VB−0.94VC0.89VD−0.89VIf an element can exist in several oxidation states, it is convenient to display the reduction potentials corresponding to the various half reactions in diagrammatic from, known as Latimer diagram. The Latimer diagram for chlorine in acid solution is
Cl−4+1.20V−−−−→ClO−3+1.60V−−−−→ClO−2+1.60V−−−−→
ClO−+1.67V−−−−→Cl2+1.36V−−−−→Cl−
In basic solution is :
ClO−4+0.37V−−−−→ClO−3+0.30V−−−−→ClO−2+0.68−−−→
ClO−+0.42V−−−−→Cl2+1.36−−−→Cl−. The standard potentials for two nonadjacent species can also be calculated by using the concept that ΔG∘ is an additive property but using potential is not an assitive property and ΔG∘=−nFx0, If a given oxidation state is a the next higher oxidation state disproportionation can occur. The reverse of relative stabilities of the oxidation state can also be understood by drawing a graph of ΔG∘/F against oxidation state, known as Frost diagram, Choosing the stability of zero oxidation state arbitrery as zero. The most stable oxidation state of a apecies lies lowest in the digram Disproportionation is spontaneous if the species lies above a straight line joining its two product species.
Which of the following couple have same value of potential at pH = 0 and pH = 14?AClO−4ClO−3BClO−2Cl2CClO−Cl2DCl2Cl−If an element can exist in several oxidation states, it is convenient to display the reduction potentials corresponding to the various half reactions in diagrammatic from, known as Latimer diagram. The Latimer diagram for chlorine in acid solution is
Cl−4+1.20V−−−−→ClO−3+1.60V−−−−→ClO−2+1.60V−−−−→
ClO−+1.67V−−−−→Cl2+1.36V−−−−→Cl−
In basic solution is :
ClO−4+0.37V−−−−→ClO−3+0.30V−−−−→ClO−2+0.68−−−→
ClO−+0.42V−−−−→Cl2+1.36−−−→Cl−. The standard potentials for two nonadjacent species can also be calculated by using the concept that ΔG∘ is an additive property but using potential is not an assitive property and ΔG∘=−nFx0, If a given oxidation state is a the next higher oxidation state disproportionation can occur. The reverse of relative stabilities of the oxidation state can also be understood by drawing a graph of ΔG∘/F against oxidation state, known as Frost diagram, Choosing the stability of zero oxidation state arbitrery as zero. The most stable oxidation state of a apecies lies lowest in the digram Disproportionation is spontaneous if the species lies above a straight line joining its two product species.
For a hypothetical element, the Frost diagram is shown in figure
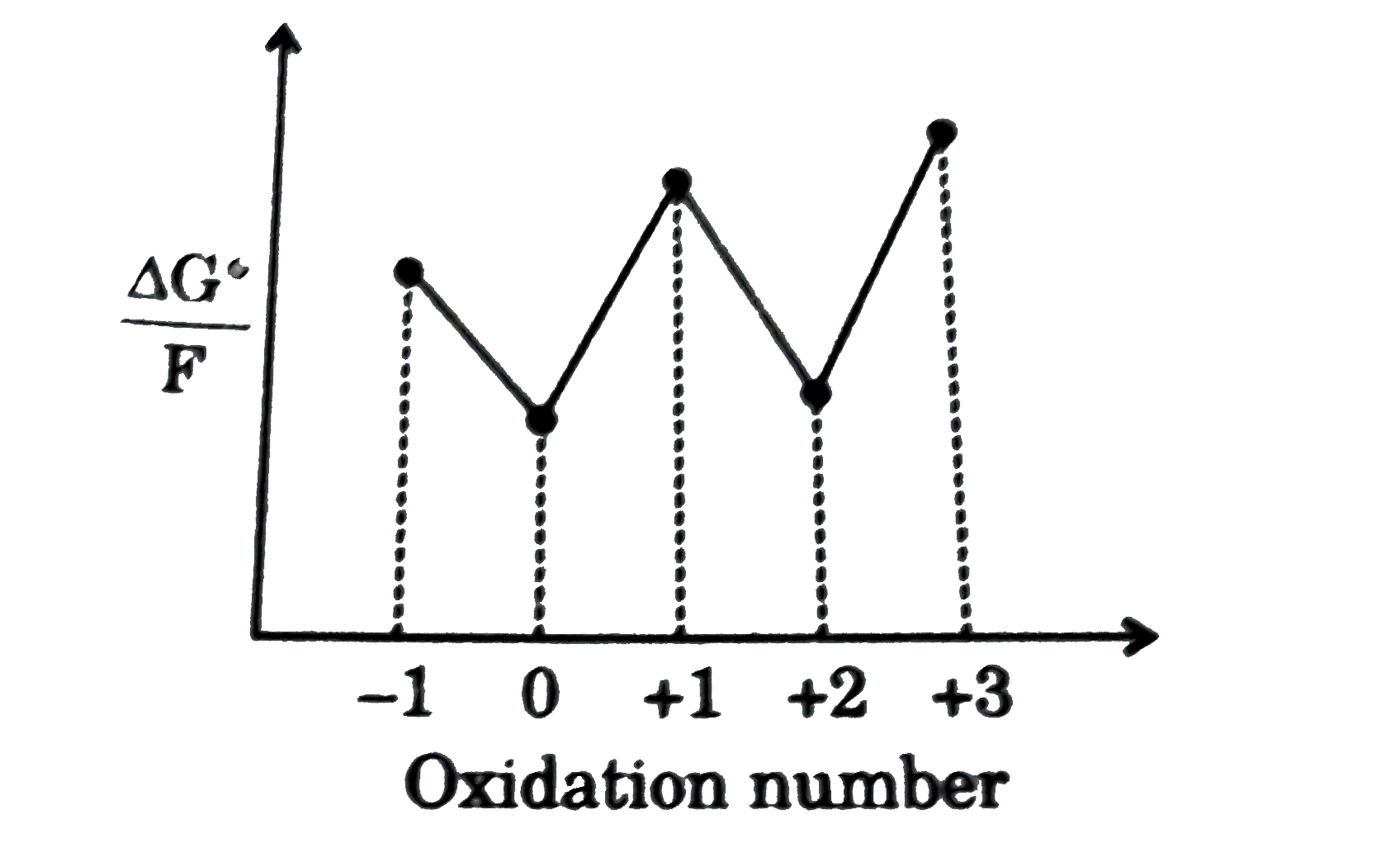
Which of the following oxidation state is least stable ?A-1B0C2D3
If an element can exist in several oxidation states, it is convernient to display the reduction potentials correspondingg to the various half reactions in diagrammatic form, know as latimer diagram the latimer diagram for chlorine in acid solutio is
CIO−4+1.20V−−−−→CiO−3+1.18V−−−−→HClO2+1.60V−−−−→HClO1.67V−−→Cl21.36V−−→Cl− in basic solution.
ClO−40.37V−−→ClO−30.30V−−→ClO−20.68V−−→ClO−0.42V−−→Cl21.36V−−→Cl− The standard potentials for two nonadjacent species can also be calculateed by using the concept that △G∘ as an additive property but potential is not an additive property and △G∘=−nFx0. if a given oxidation stateis a stronger oxidising agent than the next higher oxidation state, disproportionation can occur. The reverse of disproportionation is called comproportionation. The relative stabilities of the oxidation state can also be understood by drawing a graph of △G∘/F against oxidation state, known as frost diagram, choosing the stability of zero oxidation state arbitrarily as zero. The most stable oxidation state of a species lies lowest in the diagram, disproportionation is spontaneous if the species lies above a straight line joining its two product species.
Q. Which of the following couple have same value of potential at pH=0 and pH=14?
View SolutionIf an element can exist in several oxidation states, it is convernient to display the reduction potentials correspondingg to the various half reactions in diagrammatic form, know as latimer diagram the latimer diagram for chlorine in acid solutio is
CIO−4+1.20V−−−−→CiO−3+1.18V−−−−→HClO2+1.60V−−−−→HClO1.67V−−→Cl21.36V−−→Cl− in basic solution.
ClO−40.37V−−→ClO−30.30V−−→ClO−20.68V−−→ClO−0.42V−−→Cl21.36V−−→Cl− The standard potentials for two nonadjacent species can also be calculateed by using the concept that △G∘ as an additive property but potential is not an additive property and △G∘=−nFx0. if a given oxidation stateis a stronger oxidising agent than the next higher oxidation state, disproportionation can occur. The reverse of disproportionation is called comproportionation. The relative stabilities of the oxidation state can also be understood by drawing a graph of △G∘/F against oxidation state, known as frost diagram, choosing the stability of zero oxidation state arbitrarily as zero. The most stable oxidation state of a species lies lowest in the diagram, disproportionation is spontaneous if the species lies above a straight line joining its two product species.
Q. Which of the following statement is correct?
View SolutionIf an element can exist in several oxidation states, it is convernient to display the reduction potentials correspondingg to the various half reactions in diagrammatic form, know as latimer diagram the latimer diagram for chlorine in acid solutio is
CIO−4+1.20V−−−−→CiO−3+1.18V−−−−→HClO2+1.60V−−−−→HClO1.67V−−→Cl21.36V−−→Cl− in basic solution.
ClO−40.37V−−→ClO−30.30V−−→ClO−20.68V−−→ClO−0.42V−−→Cl21.36V−−→Cl− The standard potentials for two nonadjacent species can also be calculateed by using the concept that △G∘ as an additive property but potential is not an additive property and △G∘=−nFx0. if a given oxidation stateis a stronger oxidising agent than the next higher oxidation state, disproportionation can occur. The reverse of disproportionation is called comproportionation. The relative stabilities of the oxidation state can also be understood by drawing a graph of △G∘/F against oxidation state, known as frost diagram, choosing the stability of zero oxidation state arbitrarily as zero. The most stable oxidation state of a species lies lowest in the diagram, disproportionation is spontaneous if the species lies above a straight line joining its two product species.
Q. Which of the following statement is correct?
View SolutionIf an element can exist in several oxidation states, it is convernient to display the reduction potentials correspondingg to the various half reactions in diagrammatic form, know as latimer diagram the latimer diagram for chlorine in acid solutio is
CIO−4+1.20V−−−−→CiO−3+1.18V−−−−→HClO2+1.60V−−−−→HClO1.67V−−→Cl21.36V−−→Cl− in basic solution.
ClO−40.37V−−→ClO−30.30V−−→ClO−20.68V−−→ClO−0.42V−−→Cl21.36V−−→Cl− The standard potentials for two nonadjacent species can also be calculateed by using the concept that △G∘ as an additive property but potential is not an additive property and △G∘=−nFx0. if a given oxidation stateis a stronger oxidising agent than the next higher oxidation state, disproportionation can occur. The reverse of disproportionation is called comproportionation. The relative stabilities of the oxidation state can also be understood by drawing a graph of △G∘/F against oxidation state, known as frost diagram, choosing the stability of zero oxidation state arbitrarily as zero. The most stable oxidation state of a species lies lowest in the diagram, disproportionation is spontaneous if the species lies above a straight line joining its two product species.
Q. What is the potential couple ClO−Cl− at pH=14?
View SolutionIf an element can exist in several oxidation states, it is convernient to display the reduction potentials correspondingg to the various half reactions in diagrammatic form, know as latimer diagram the latimer diagram for chlorine in acid solutio is
CIO−4+1.20V−−−−→CiO−3+1.18V−−−−→HClO2+1.60V−−−−→HClO1.67V−−→Cl21.36V−−→Cl− in basic solution.
ClO−40.37V−−→ClO−30.30V−−→ClO−20.68V−−→ClO−0.42V−−→Cl21.36V−−→Cl− The standard potentials for two nonadjacent species can also be calculateed by using the concept that △G∘ as an additive property but potential is not an additive property and △G∘=−nFx0. if a given oxidation stateis a stronger oxidising agent than the next higher oxidation state, disproportionation can occur. The reverse of disproportionation is called comproportionation. The relative stabilities of the oxidation state can also be understood by drawing a graph of △G∘/F against oxidation state, known as frost diagram, choosing the stability of zero oxidation state arbitrarily as zero. The most stable oxidation state of a species lies lowest in the diagram, disproportionation is spontaneous if the species lies above a straight line joining its two product species.
Q.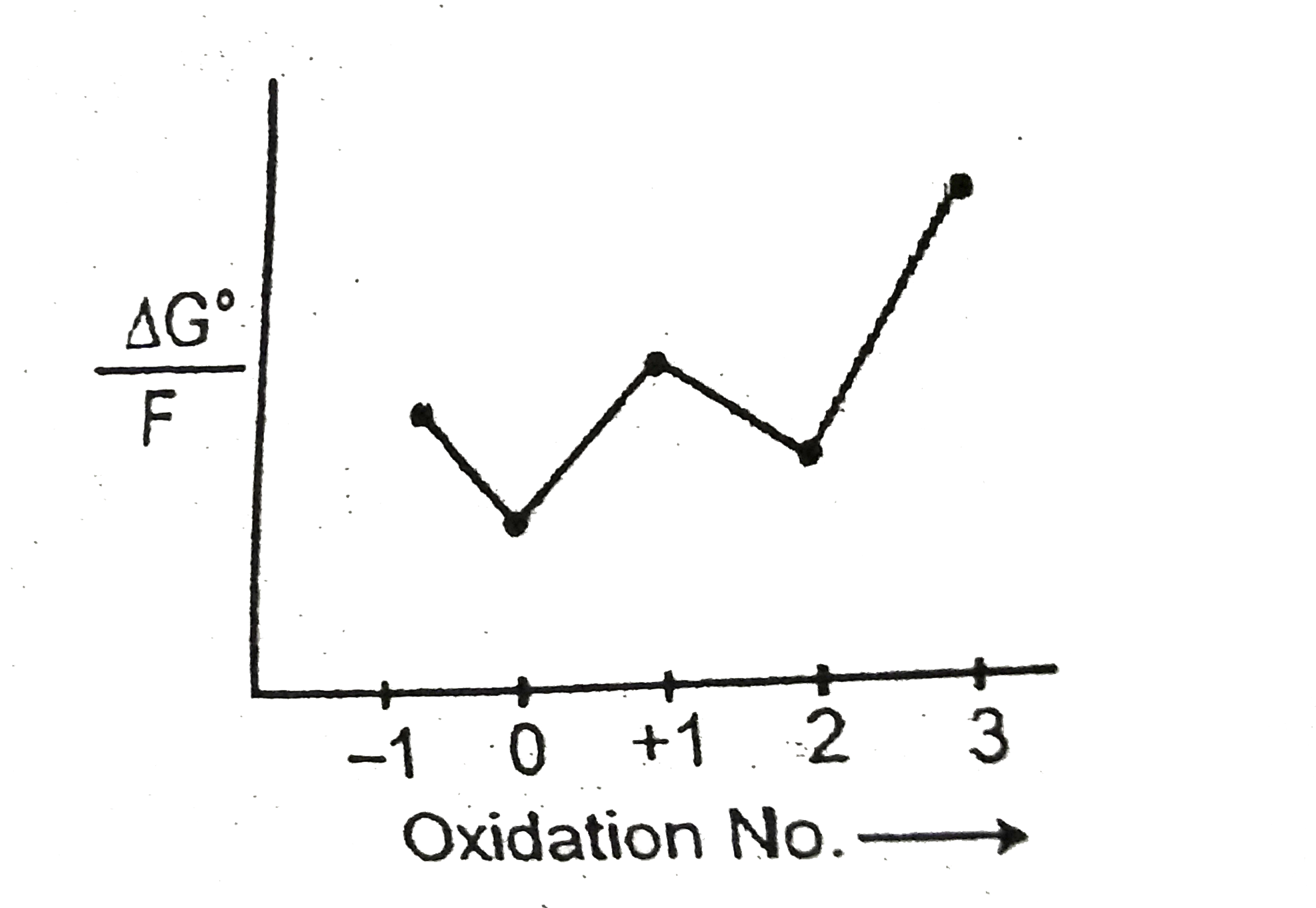
For a hypothetical element, the frost diagram is shown in figure.? which of the following oxidation state is least stable?
View Solution

