One mole of an ideal monoatomic gas is heated according to path AB and AC. If temperature of state B and C are equal then calculate qACqAB×10.
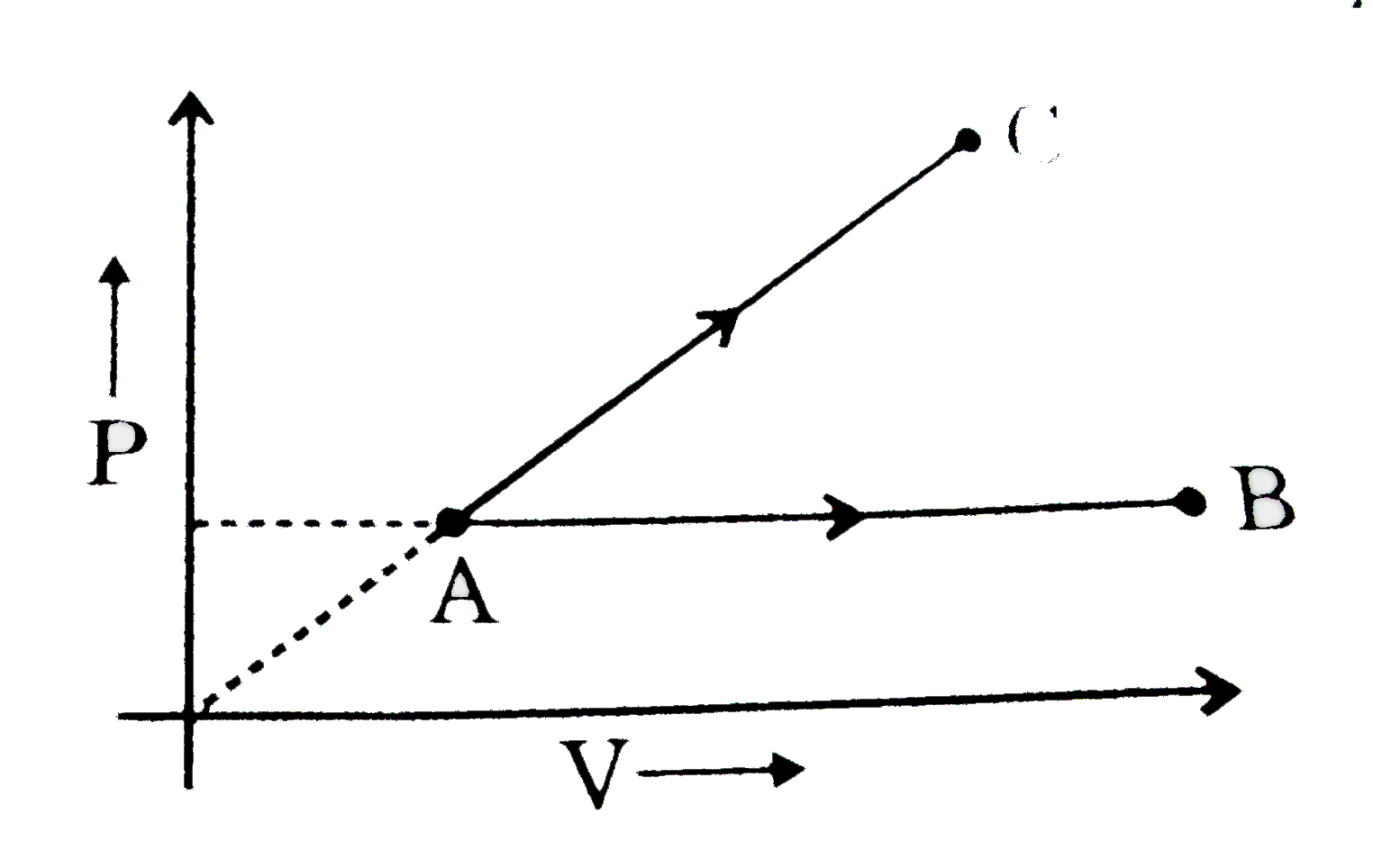
The correct Answer is:8
Similar Questions
One mole ideal monoatomic gas is heated according to path AB and AC.
If temperature of state B and state C are equal.
Calculate qACqAB×10.
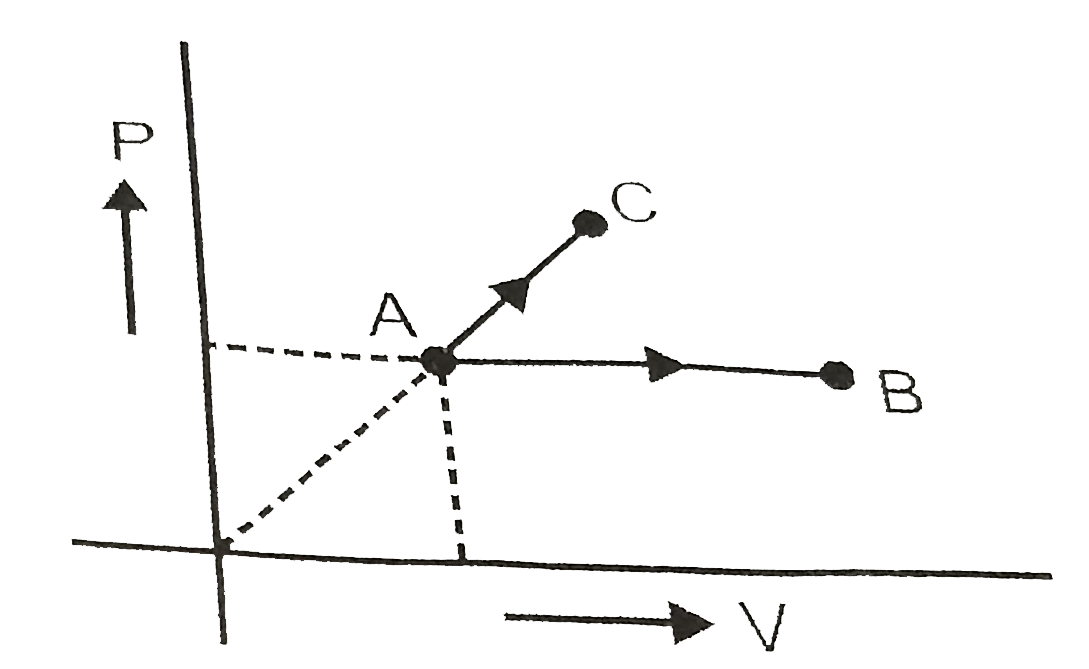
View SolutionOne mole of an ideal monoatomic gas is taken along the path ABCA as shown in the PV diagram. The maximum temperature attained by the gas along the path BC is given by :

View Solution
Knowledge Check
One mole of an ideal and monoatomic gas is expanded reversibly and isothermally at 298K temperature according to the given diagram, calculate work done.
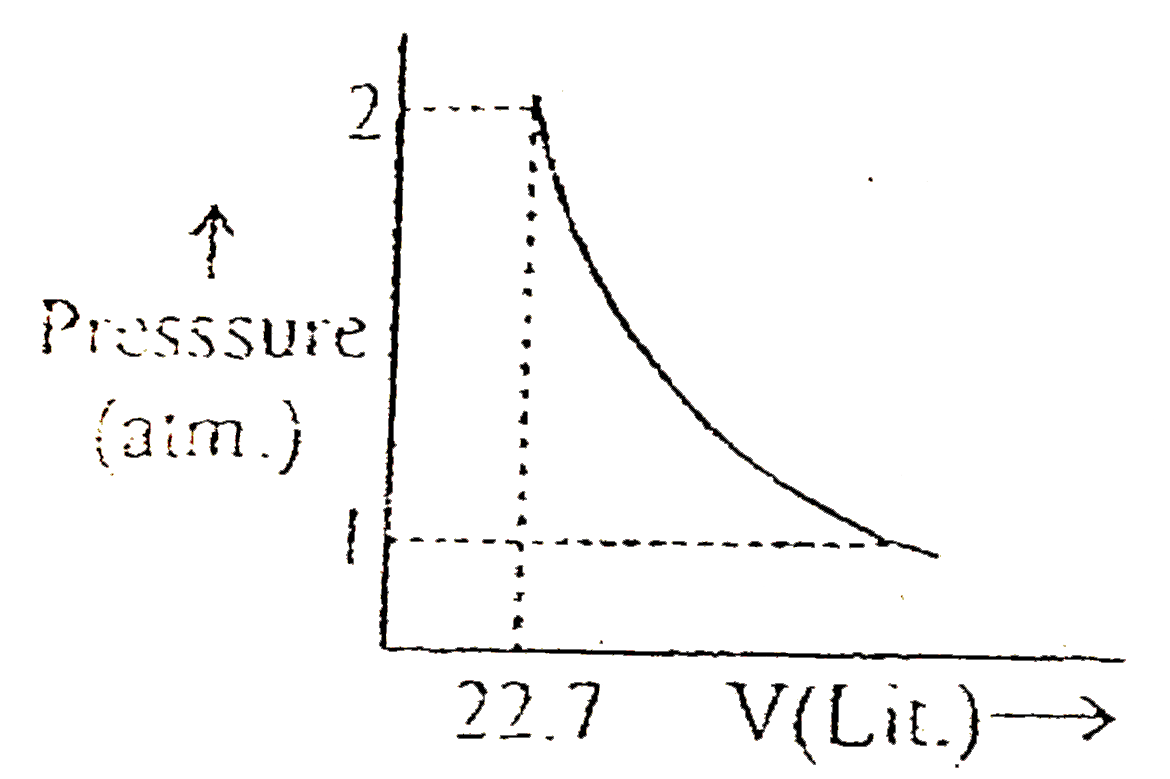 A−1717.46JB+1717.46JC−171.746JD+171.746J
A−1717.46JB+1717.46JC−171.746JD+171.746JOne mole of an ideal monoatomic gas at temperature T0 expands slowely according to the law P/V=constant. If the final temperature is 2T0 heat supplied to the gas is -
A2RT0B32RT0CRT0D12RT0One mole of an ideal monoatomic gas at temperature T0 expands slowly according to the law pV = constant. If the final temperature is 2T0, heat supplied to the gas is
A2RT0B32RT0CRT0D12RT0
One mole of an ideal monoatomic gas is taken along the path ABCA as shown in the PV diagram. The maximum temperature attained by the gas along the path BC is given by :

View SolutionOne mole of an ideal and monoatomic gas is expanded reversibly and isothermally at 298K temperature according to the given diagram, calculate work done.
View SolutionOne mole of an ideal monoatomic gas at temperature T0 expands slowly according to the law pV = constant. If the final temperature is 2T0, heat supplied to the gas is
View SolutionOne mole of an ideal monoatomic gas at temperature T0 expands slowly according to the law pV = constant. If the final temperature is 2T0, heat supplied to the gas is
View SolutionOne mole of an ideal monatomic gas at temperature T0 expands slowly according to the law PV=constant.If the final temperature is 2T0, heat supplied to the gas is
View Solution

