Between two isotherms we have a cycle as shown. Find the work done by the gas during the cycle in (J). [Take: T1=127∘C,T2=16∘C,n=1 mole]
![Between two isotherms we have a cycle as shown. Find the work done by the gas during the cycle in (J). [Take: T(1) = 127^(@)C, T(2) = 16^(@)C, n = 1 mole]](https://d10lpgp6xz60nq.cloudfront.net/physics_images/GRB_PHY_CHM_P2_V03_QB_C13_E01_430_Q01.png)
Text Solution
The correct Answer is:75
|
Similar Questions
Knowledge Check
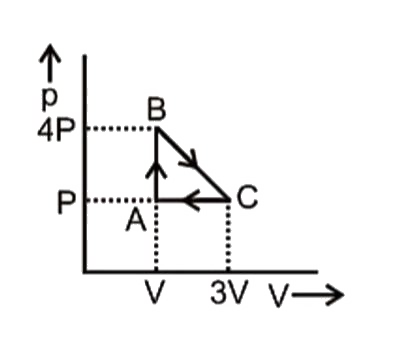
An ideal gas is taken around the cycle ABCA as shown in the P-V diagram.
The total work done by the gas during the cycle is
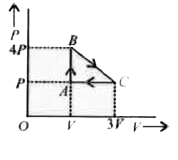 APVB2PVC4PVD3PV
APVB2PVC4PVD3PVAn ideal gas is taken around the cycle ABCD A as shown in figure . The net work done during the cycle
is equal to :
 AzeroBpositiveCnegativeDwe cannot predict
AzeroBpositiveCnegativeDwe cannot predictTwo moles of helium gas are taken over the cycle ABCDA , as shown in the P-Tdigram

The net work done on the gas in the cycle ABCD A is -.AZeroB−276RC1076RD1904R
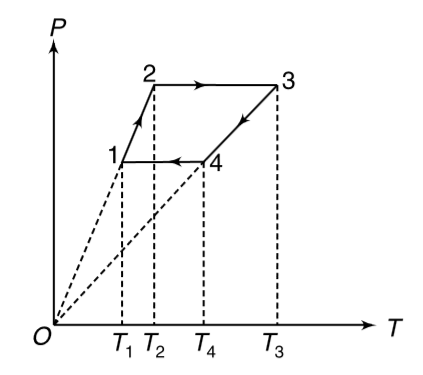
n moles of an ideal gas is taken through a four step cyclic process as shown in the diagram. Calculate work done by the gas in a cycle in terms of temperatures T1,T2,T3 and T4
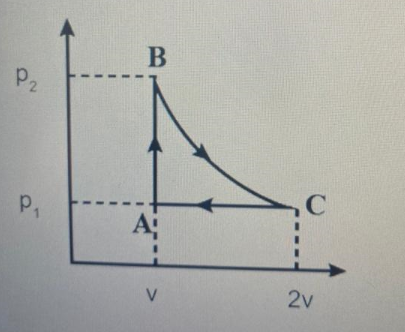
View SolutionIn a cyclic process ABCA then process BC is isothermal. Find work done by gas in cycle ABCA (Given P1=P)
View SolutionAn ideal monatomic gas is taken round the cycle ABCDA as shown in the P-V diagram (see figure). The work done during the cycle is

View SolutionAn ideal monoatomic gas is taken round the cycle ABCDA as shown in the P-V diagram. The work done during the cycle is
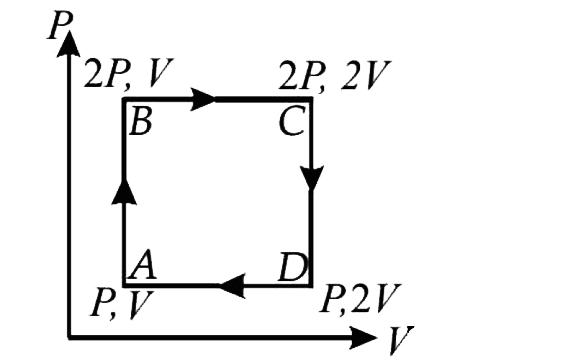
View SolutionAn ideal monoatomic gas is taken round the cycle ABCDA as shown in the P-V diagram. The work done during the cycle is

View Solution