Reactions involving heterolytic fission are said to be produced via
A
Ionic mechanism
B
Polar mechanism
C
Both (a) and (b)
D
None of these
Text Solution
The correct Answer is:C
|
Topper's Solved these Questions
- BASIC PRINCIPLES AND TECHNIQUES IN ORGANIC CHEMISTRYBook:MARVEL PUBLICATIONChapter:BASIC PRINCIPLES AND TECHNIQUES IN ORGANIC CHEMISTRYExercise:Test your grasp
Explore 40 Videos
- BASIC CONCEPT OF CHEMISTRYBook:MARVEL PUBLICATIONChapter:BASIC CONCEPT OF CHEMISTRYExercise:TEST YOUR GRASP
Explore 50 Videos
- NATURE OF CHEMICAL BOND Book:MARVEL PUBLICATIONChapter:NATURE OF CHEMICAL BOND Exercise:TEST YOUR GRASP
Explore 50 Videos
Similar Questions
Knowledge Check
Convalent bond can undergo fission in two different ways. The correct representation involving a heterolytic fission of CH3−Br is
A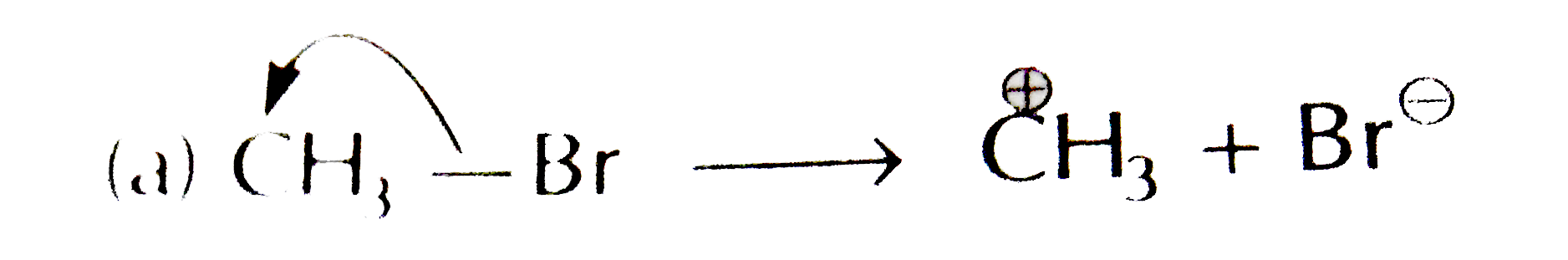 B
B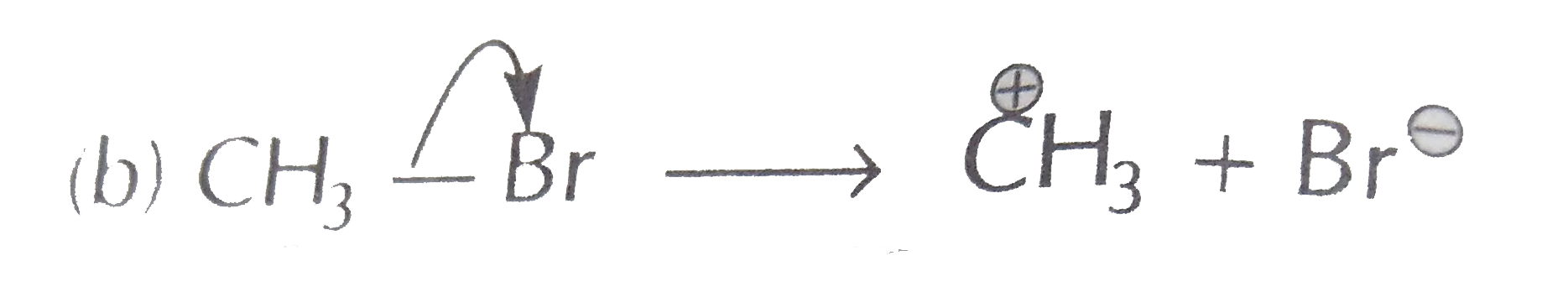 C
C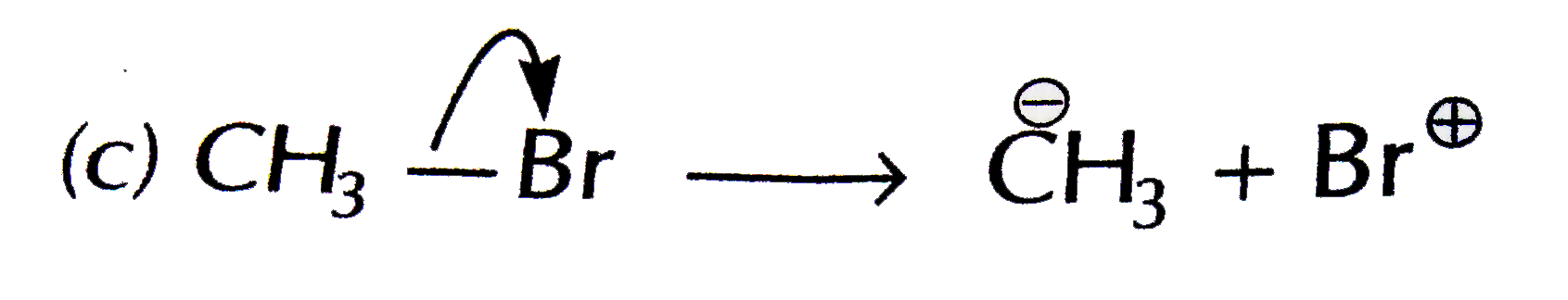 D
D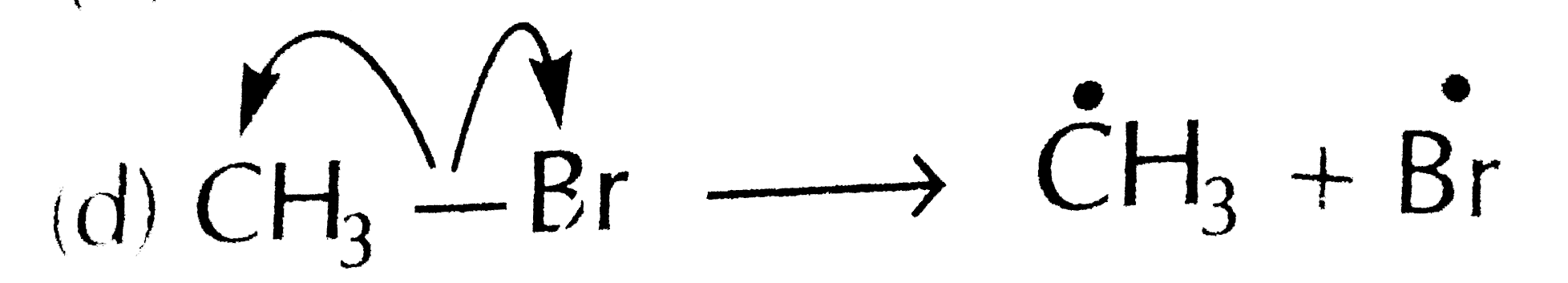
Convalent bond can undergo fission in two different ways. The correct representation involving a heterolytic fission of CH3−Br is
A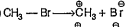 B
B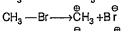 C
C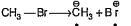 D
D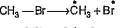
Covalent bond undergo fission in two different ways. The correct representation involving a heterolytic fission of CH3−Br is
ACH3−Br→⊕CH3+BrɵBCH3−Br→⊕CH3+BrɵCCH3−Br→ɵCH3+BrɵDCH3−Br→⋅CH3+Br⋅
Reaction happens via

View SolutionReaction happens via

View Solution
Reaction happens via
View SolutionCovalent bond can undergo fission in two different ways. The correct represenation involving a heterolytic fission of CH3−Br is
View SolutionHeterolytic fission gives rise to ________.
View Solution

