How much heat should be supplied to convert ice of mass 250 g at 0∘C into water at 20∘C?
25,000 cal
Topper's Solved these Questions
- HEATBook:TARGET PUBLICATIONChapter:HEATExercise:Apply your Knowledge
Explore 36 Videos
- HEATBook:TARGET PUBLICATIONChapter:HEATExercise:Chapter Assessment
Explore 18 Videos
- HEATBook:TARGET PUBLICATIONChapter:HEATExercise:Solve the following problems
Explore 22 Videos
- GRAVITATIONBook:TARGET PUBLICATIONChapter:GRAVITATIONExercise:ASSESSMENT
Explore 19 Videos
- LENSES Book:TARGET PUBLICATIONChapter:LENSES Exercise:Chapter Assessment
Explore 21 Videos
Similar Questions
Knowledge Check
How much heat is reuired to convert 5.0 g of ice at −10.0∘ C to liquid water at 15.0∘ C ? (Assume heart capacities are indendent o ftemperature.)
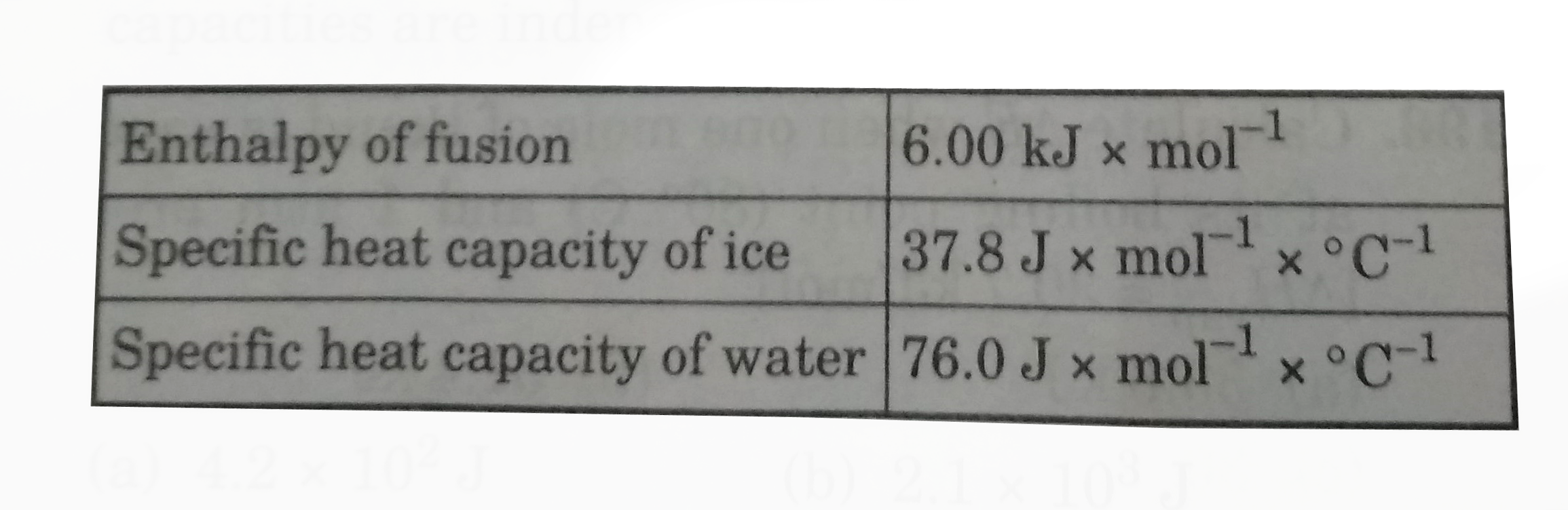 A4.2×102jB2.1×103JC9.3×103JD3.8×104J
A4.2×102jB2.1×103JC9.3×103JD3.8×104JHeat required to convert 1 g of ice at 0∘C into steam at 100∘C is
A100 calB0.01cal/∘CC720 calD1 kilocal
Find the amount of heat required to convert 10g of water at 100∘C into steam. (Spefic latent heat of vaporization of water =540cal/g )
View SolutionCalculate the amount of heat energy to be supplied to convert 5 kg of ice at 0∘C completely into water at 0∘C if latent heat of melting ice is 80 cal/g.
View SolutionCalculate the amount of heat required to convert5g of ice of 0∘C into water at 0∘C.
( Specific latent heat of fusion of ice 80cal/g )
View SolutionHow much heat is required to convert 1 kg ice at 0∘C to steam at 100∘C ?
View SolutionCalculate the amount of heat energy to be supplied to convert 4 kg of ice at 0∘C completely into water at0∘C if latent heat of fusion for ice is 80 cal/g.
View SolutionCalculate the amount of heat energy to be supplied to convert 2 kg of ice at 0∘C completely into water at0∘C if latent heat of fusion for ice is 80 cal/g.
View Solution

