How is electron-dot structure of a molecule drawn ? Explain giving example.
Video Solution
Text Solution
i. The electronic configuration of carbon is (2, 4). It has four electrons in its valence shell.
ii. It could form C4+ cation to attain the stable duplet configuration of noble gas helium (He) by losing one after another all th efour valence electrons. Valence electrons can be represented as dots or corsses.
ii. Now, show a covalent bond between atoms by overlapping the circles. Shared electrons are shown in the overlapping region of the two circles.
iii. Electron - dot structure can also be drawn without showing circles.
E.g. Electron-dot dtructure of H2 molecules with circles :

Electron-dot structure of H2 molecules without circles :
H:H
|
Similar Questions
Knowledge Check
Draw the electron dot structures for
Aethanoic acidBH2SCpropanoneDF2The correct electron dot structure of a water molecule is
AH.⋅⋅O⋅⋅.HBH:⋅⋅O⋅⋅.HCH:⋅⋅O⋅⋅:HDH:O:HThe correct electron dot structure of a water molecule is
A B
B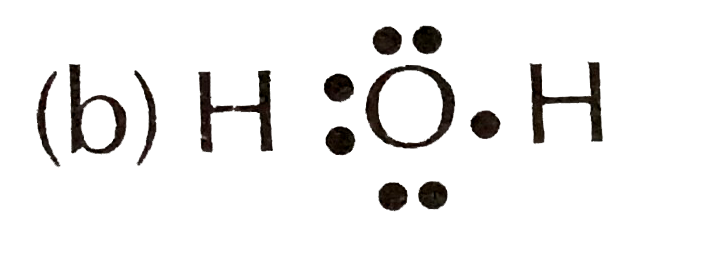 C
C D
D

