The correct order of S-S bond length in following oxyanions is:
S2O2−4
(II) S2O2−5
(III) S2O2−6
A
I>II>III
B
I>III>II
C
III>II>I
D
III>I>II
Text Solution
The correct Answer is:A
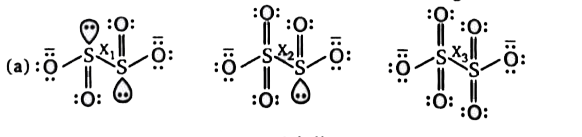
According to Bents rule x1>x2>x3.
|
Topper's Solved these Questions
- p-BLOCK ELEMENTSBOOK - VK JAISWALCHAPTER - p-BLOCK ELEMENTSEXERCISE - LEVEL 3 (PASSAGE TYPE)
20 Videos
- p-BLOCK ELEMENTSBOOK - VK JAISWALCHAPTER - p-BLOCK ELEMENTSEXERCISE - ONE OR MORE ANSWERS IS/ARE CORRECT
97 Videos
- p-BLOCK ELEMENTSBOOK - VK JAISWALCHAPTER - p-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
36 Videos
- METALLURGYBOOK - VK JAISWALCHAPTER - METALLURGYEXERCISE - ASSERTION-REASON TYPE QUESTIONS
30 Videos
- PERIODIC PROPERTIESBOOK - VK JAISWALCHAPTER - PERIODIC PROPERTIESEXERCISE - ASSERTION-REASON TYPE QUESTIONS
35 Videos
Similar Questions
Knowledge Check
The correct order of S-S bod length is following oxyanions is :
(i) S2O2−4 (ii) S2O2−5 (iii) S2O2−6AI>II>IIIBI>III>IICIII>II>IDIII>I>III2+S2O2−3→I−+S4O2−6
AFor disproportionation reaction.BFor comproportionation reaction.CFor either intermolecular redox reaction or displacement reactionDFor either thermal combination redox reaction or thermal decomposition redox reaction.I2+S2O2−3→I−+S4O2−6
AFor disproportionation reaction.BFor comproportionation reaction.CFor either intermolecular redox reaction or displacement reactionDFor either thermal combination redox reaction or thermal decomposition redox reaction.
The correct order of sulphur - oxygen bond energy in S2O2−3,SO2−4,SO3andS2O2−6 is
View SolutionWhat is the correct order of oxidation states of suphur in anions So2−3,S2O2−4 and S2_(6)2− ?
View SolutionIdentify the correct sequence of increasing number of π-bonds in the structure of the following molecules:
(I)H2S2O6 (II)H2S2O3 (III)H2S2S5
View SolutionIdentify the correct sequence of increasing number of π-bonds in the structure of the following molecules:
(I)H2S2O6 (II)H2S2O3 (III)H2S2S5
View SolutionIdentify the correct sequence of increasing of π− bonds in the structure of the following molecules :
(I)H2S2O6 (II)H2S2O3 (III)H2S2O5
View Solution

