How many moles of NaOH are required to react with one mole of solid N2O5?
The correct Answer is:2
Step by step video, text & image solution for How many moles of NaOH are required to react with one mole of solid N_(2)O_(5)? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Calculate the number of moles of Cr2O2−7 required to oxidise 1 " mol of "Fe(HC2O4) in acidic medium. How many moles of NaOH are required to react with 1 " mol of "Fe(HC2O4)?
View SolutionPotassium iodide reacts with acidified K2Cr2O7. How many moles of KI are required for one mole of K2Cr2O7 ?
View Solution
Knowledge Check
How many moles of KCIO3 are required for getting 30 mol of O2 ?
A40B30C20D10How many moles of KCIO3 are required for getting 30 mol of O2?
A40B30C20D10How many moles of KI are required to produce 0.4 moles of K2HgI4 ?
A0.4B0.8C3.2D1.6
Urea reacts with Br2 in dil HCl to liberate N2 How many moles of bromine is required to completely react with one mole of urea ?
View SolutionHow many moles of NaOH is required to neutralize 1 mole of H3PO3
View SolutionHow many moles of KMnO4 will be required to react completely with 1 " mol of "K2C2O4 (potassium oxalate) in acidic medium?
View Solution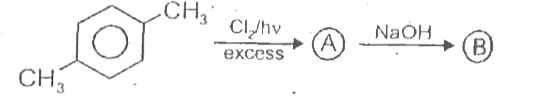
How many moles of CH3OH required to react completely with (B) ?
View SolutionHow many moles of KMnO4 are required to oxidise one mole of SnCl2 in acidic medium?
View Solution

