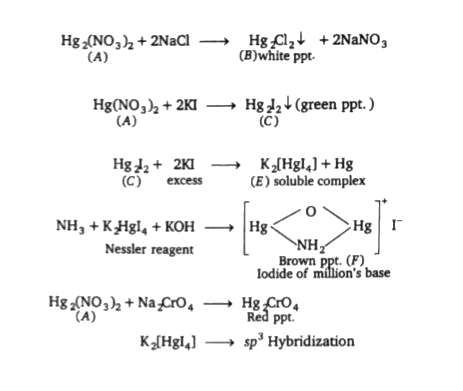
(A)+NaCl→(B) (white ppt.)
(B)+Ki→(C) (green ppt).
(C)+KI(excess)→(D)+(E) (colourless solution)
(E)+NH3+KOH→(F)
Q. Compounds (A) and (B) are respectively:
AgNO3andAgCl
Pb(NO3)2andPbCl2
Hg2(NO3)2andHg2Cl2
Cu2(NO3)2andCu2Cl2
The correct Answer is:C
Similar Questions
(A)+NaCl to (B) (white ppt.) (B)+Kito(C) (green ppt). (C)+underset(("excess"))(KI) to (D)+(E) (colourless solution) (E)+NH_(3)+KOH to (F) Q. Type of hybridization in compound (E) is:
View Solution(A)+NaCl→(B) (white ppt.)
(B)+Ki→(C) (green ppt).
(C)+KI(excess)→(D)+(E) (colourless solution)
(E)+NH3+KOH→(F)
Q. Colour of the compound (F) is:
View SolutionLight green (compound 'A') overset(Delta)(to)"white Residue(B)"overset("high")underset("Temp.")C+D+E (i)'D' and 'E' are two acidic gas. (ii) 'D' is passed through HgCl_(2) solution to give yellow ppt. (iii) 'E' is passed through water first and then H_(2)S is passed, white turbidity is obtained. (iv) A is water soluble and addition of HgCl_(2) in it, yellow ppt. is obtained but white ppt does not turn into grey on addition of excess solution of 'A' Q. 'C' is soluble in :
View Solution(A)+NaCl→(B) (white ppt.)
(B)+Ki→(C) (green ppt).
(C)+KI(excess)→(D)+(E) (colourless solution)
(E)+NH3+KOH→(F)
Q. Compounds (A) and (B) are respectively:
View Solution(A)+NaCl to (B) (white ppt.) (B)+Kito(C) (green ppt). (C)+underset(("excess"))(KI) to (D)+(E) (colourless solution) (E)+NH_(3)+KOH to (F) Q. When compound (A) reacts with Na_(2)CrO_(4) solution, the colour of the compound formed is
View Solution(A)+NaCl→(B) (white ppt.)
(B)+Ki→(C) (green ppt).
(C)+KI(excess)→(D)+(E) (colourless solution)
(E)+NH3+KOH→(F)
Q. Type of hybridization in compound (E) is:
View Solution(A)+NaCl to (B) (white ppt.) (B)+KIto(C) (green ppt). (C)+underset(("excess"))(KI) to (D)+(E) (colourless solution) (E)+NH_(3)+KOH to (F) Q. Colour of the compound (F) is:
View Solution(A) (colourless solution ) gives white ppt (B) with NaOH solution but ppt dissolves in excess of NaOH forming (C ). (C ) does not give ppt with H_(2)S but on boiling with NH_(4)CI , white ppt (B) appears. (A) also gives yellow ppt with AgNO_(3) identify (A),(B) and (C )
View Solution