Hydrocarbon A (C7H12) was treated with BH3 .THF, H2O2,NaOH To product B(C7H14O as only product.Reaction of B with TsCI//Pydridine follwored by KOH given followied by Zn//AcOH produces only co,pounds shown below:
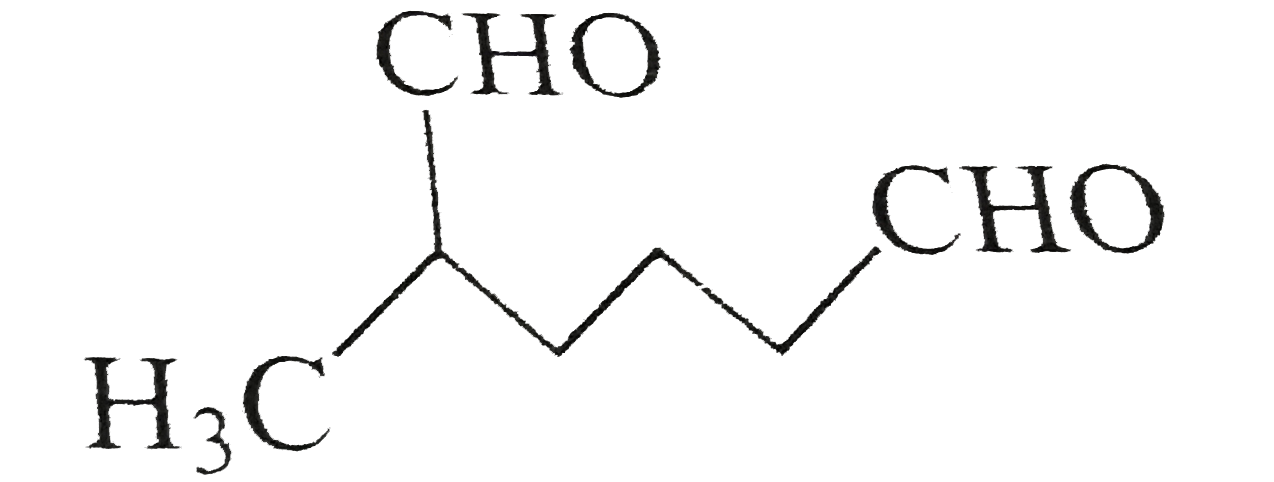
What is correct structure of 'B'?
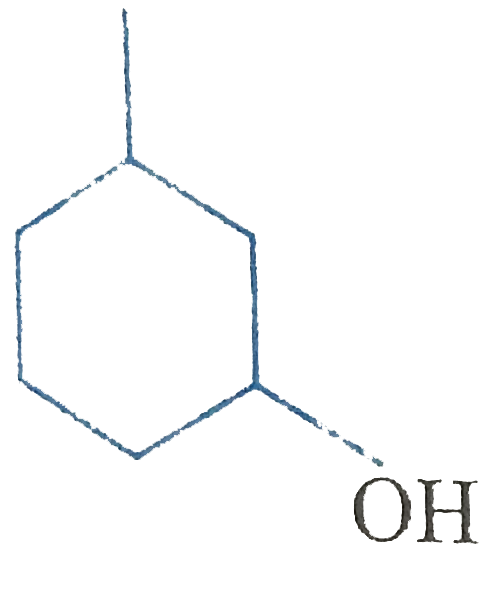
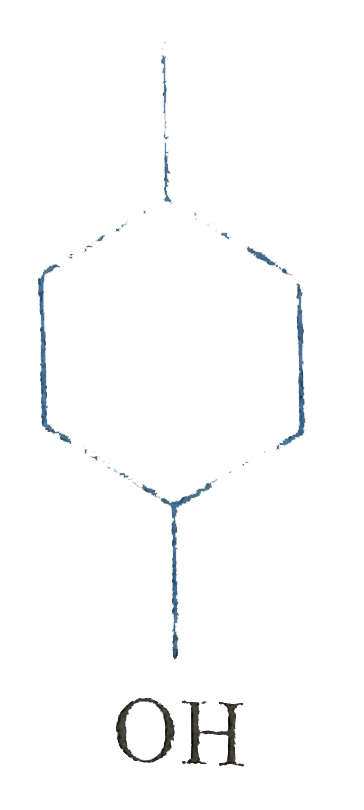
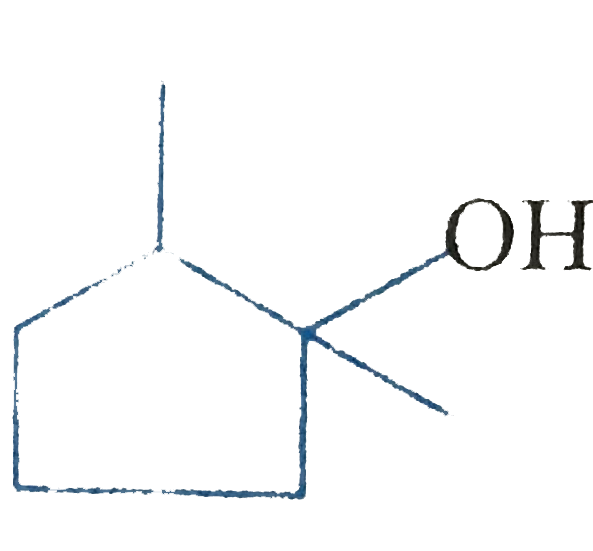
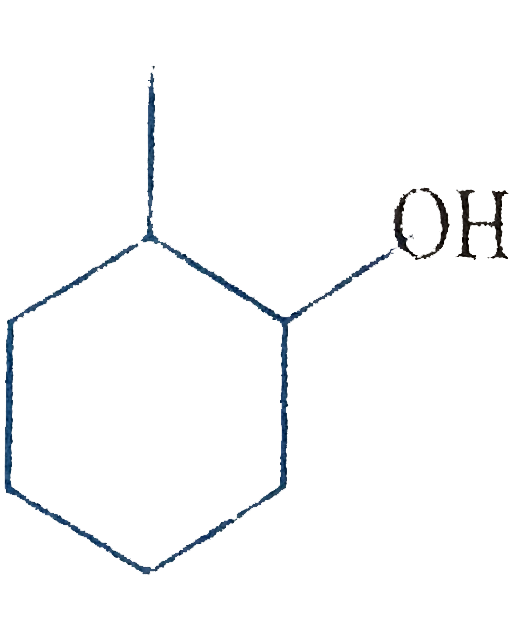
The correct Answer is:D
Step by step video, text & image solution for Hydrocarbon A (C_(7)H_(12)) was treated with BH_(3) .THF, H_(2)O_(2), NaOH To product B(C_(7)H_(14)O_() as only product.Reaction of B with TsCI//Pydridine follwored by KOH given followied by Zn//AcOH produces only co,pounds shown below: What is correct structure of 'B'? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
An organic compound (A),C7H6O gives positives test with Tollen's reagent,on treatment with alcoholic CN, (A) yields the compound (B),C14H12O2 . Compound (B) on reduction with Zn-Hg, HCl and dehyration gives an unsaturated compound (C), compound (D) , C14H12O2 . Compound (D) on heating with KOH undergoes rearrangement and subsequent acidification of rearranged products yields an acidic compound (E),C14H12O3.
Structure of compound (E) is :
View SolutionHydrocarbon A (C_(7)H_(12)) was treated with BH_(3) .THF, H_(2)O_(2), NaOH To product B(C_(7)H_(14)O_() as only product.Reaction of B with TsCI//Pydridine follwored by KOH given followied by Zn//AcOH produces only co,pounds shown below: What is correct structure of 'A'?
View SolutionHydrocarbon A (C_(7)H_(12)) was treated with BH_(3) .THF, H_(2)O_(2), NaOH To product B(C_(7)H_(14)O_() as only product.Reaction of B with TsCI//Pydridine follwored by KOH given followied by Zn//AcOH produces only co,pounds shown below: What is correct structure of 'B'?
View SolutionHydrocarbon A (C7H12) was treated with BH3 .THF, H2O2,NaOH To product B(C7H14O as only product.Reaction of B with TsCI//Pydridine follwored by KOH given followied by Zn//AcOH produces only co,pounds shown below:
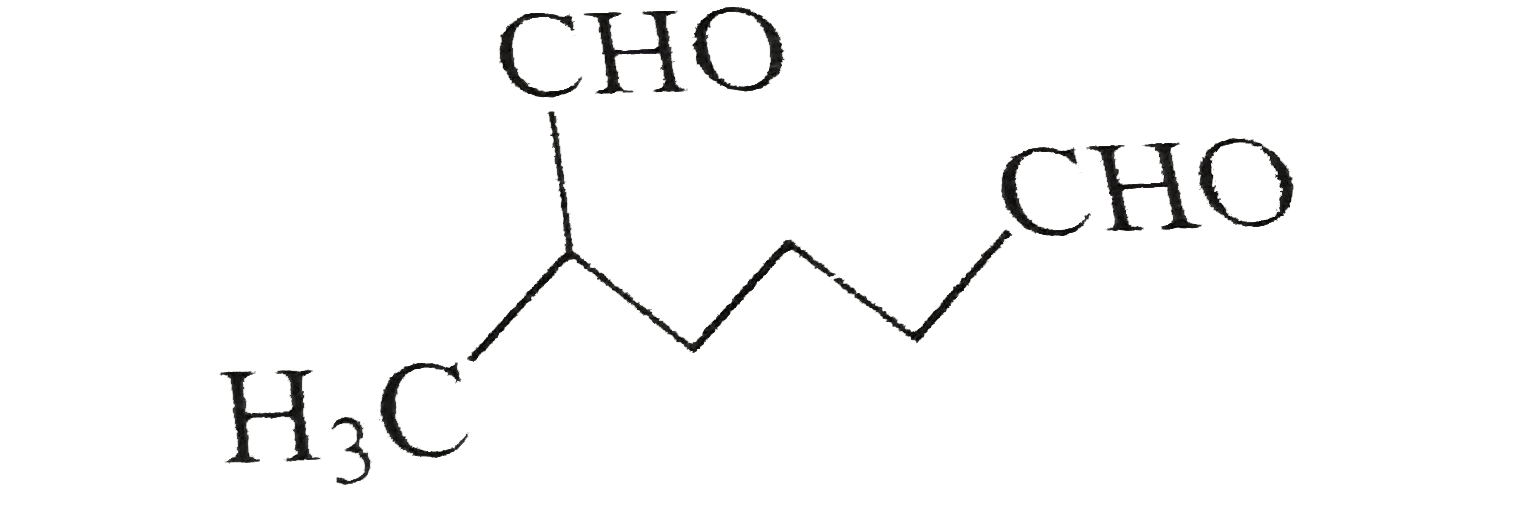
What is correct structure of compoind 'C'?
View SolutionAn organic compound A (C_(7)H_(6)Cl_(2)) on treatment with NaOH solution gives another compound B (C_(7)H_(6)O) . B on oxidation gives and acid C (C_(7)H_(6)O_(2)) which on treatment with a mixture of conc. HNO_(3)" and "H_(2)SO_(4) gives a compound D (C_(7)H_(5)NO_(4)) . B on treatment with conc. NaOH gives a compound E (C_(7)H_(8)O)" and "C_(6)H_(5)COONa . Deduce the structures of [A], [B], [C], [D] and [E].
View Solution(A) overset(O_(3))underset(Zn//AcOH)to(B) +(C ) C_(7)H_(14) Compound (A) exist in geometrical isomers and (B) Cannizaro reaction. (A) will :
View SolutionCompound (A) of molecular formula C7H8 when treated with air in presence of V2O5 at 773 K gives a compound (B) of molecular formula C7H6O, which has the smell of bitter almonds. Alkaline KMnO4 oxidises compound (B) to ( C) of molecular formula C7H6O2. Compound (B) on treatment with N2H4 and KOH gives back compound (A). Identify (A), (B) & ( C) and explain the reactions.
View SolutionC_(6)H_(12)O_(6) + Ararr2C_(3)H_(4)O_(3)+ B . A & B in the above reaction are
View Solution

