KMnO4 on treatment with conc. H2SO4 forms a compound (X) which decomposes explosively on heating forming (Y). The (X) and (Y) are respectively :
A
Mn2O7,MnO2
B
Mn2O7,Mn2O3
C
MnSO4,Mn2O3
D
Mn2O3,MnO2
Video Solution
Text Solution
The correct Answer is:A
2KMnO4+3H2SO4→2KHSO4+(MnO3)2SO4+2H2O
(MnO3)2SO4+H2O→Mn2O7+H2SO4
Mn2O7Δ−−→2MnO2+3O2
|
Similar Questions
Knowledge Check
KMnO4 in excess on treatment with concentrated H2SO4 forms a compound (X) which decomposes explosively on heating forming (Y). The (X) and (Y) are respectively.
AMn2O7,MnO2BMn2O7,Mn2O3CMnSO4,Mn2O3DMn2O3,MnO2KMnO4 is heated with conc. H2SO4 gives
AMn2O7BMnO2CMnSO4DMnOAcetone on heating with conc. H2SO4 gives :
APhoroneBAcroleinCmesityleneDmesityl oxide
Hydroazobenzene on treatment with H2SO4 forms
View Solution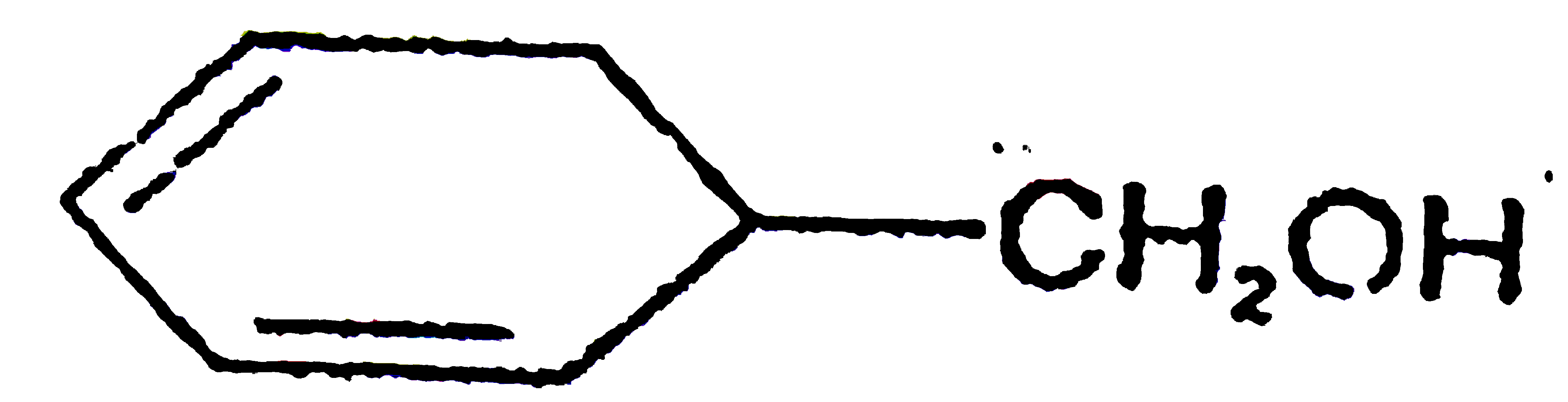 on dehydration with conc. H2SO4 predominantly forms :
on dehydration with conc. H2SO4 predominantly forms :
View SolutionMethanoic acid is heated with conc. H2SO4, to form
View SolutionCyclohexene on oxidation with conc. KMnO4 forms
View SolutionWhen ethylene glycol is treated with conc. H2SO4 the compound formed is
View Solution

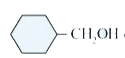 on dehydration with conc.
on dehydration with conc.