Sodium carbonate is generally prepared by a process called ammonia soda process as follows : 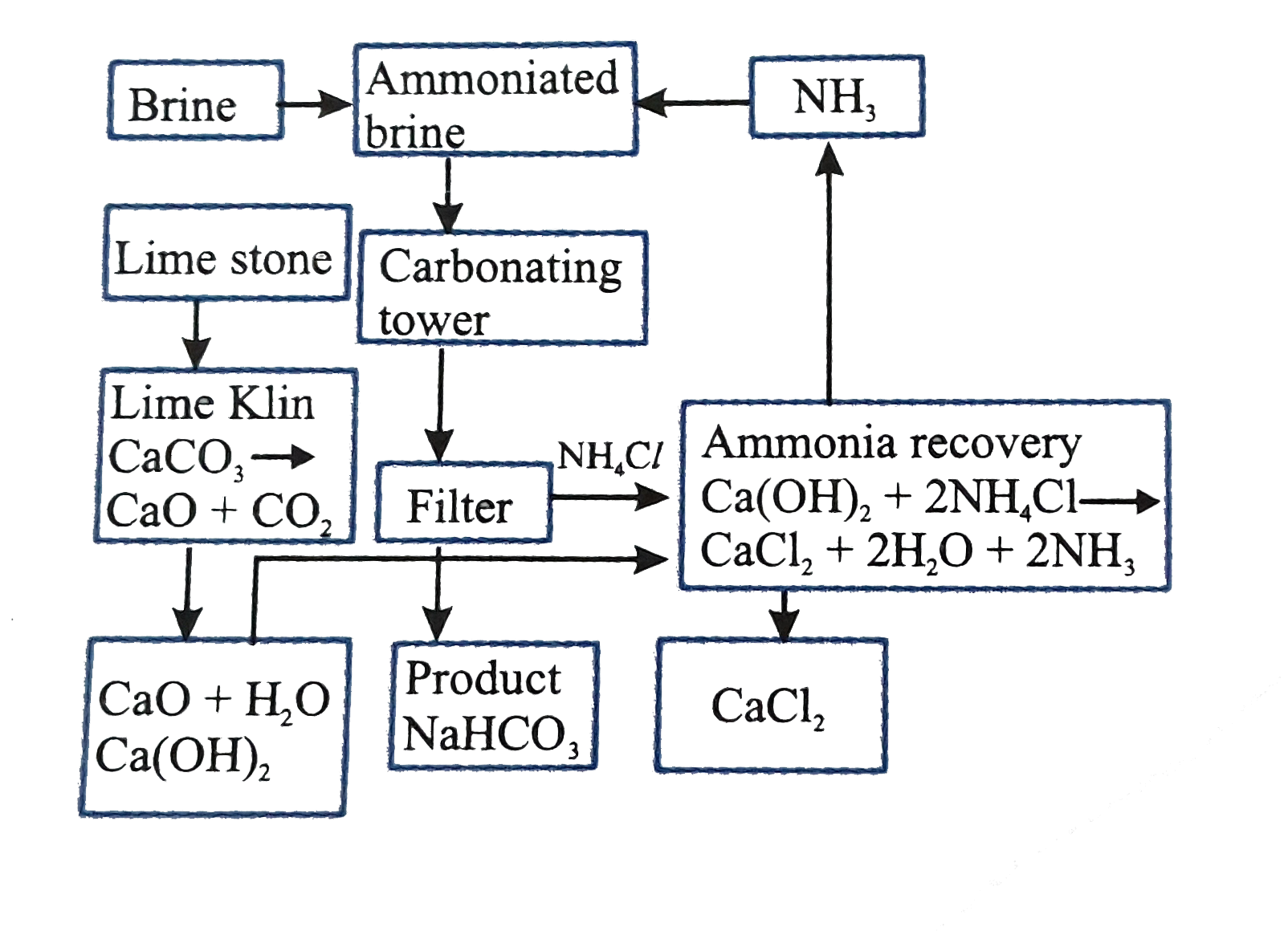
The above process cannot be used for the manufacturing of K2CO3 because
A
KHCO3 is soluble in water and doe not percipitate in carbonating water
B
KHCO3 is less soluble than NaHCO3
C
Potassium is lighter than sodium
D
Sodium reacted with water in carbonating tower
Text Solution
The correct Answer is:A
|
Topper's Solved these Questions
- S-BLOCK ELEMENTSBOOK - NARAYNACHAPTER - S-BLOCK ELEMENTSEXERCISE - Match the following
4 Videos
- S-BLOCK ELEMENTSBOOK - NARAYNACHAPTER - S-BLOCK ELEMENTSEXERCISE - ALKALINE EARTH METALS
51 Videos
- S-BLOCK ELEMENTSBOOK - NARAYNACHAPTER - S-BLOCK ELEMENTSEXERCISE - Integer
3 Videos
- REDOX REACTIONBOOK - NARAYNACHAPTER - REDOX REACTIONEXERCISE - Exercise -4
114 Videos
- SOLID STATEBOOK - NARAYNACHAPTER - SOLID STATEEXERCISE - LEVEL-V
122 Videos
Similar Questions
Knowledge Check
Sodium carbonate is generally prepared by a process called ammonia soda process as follows :
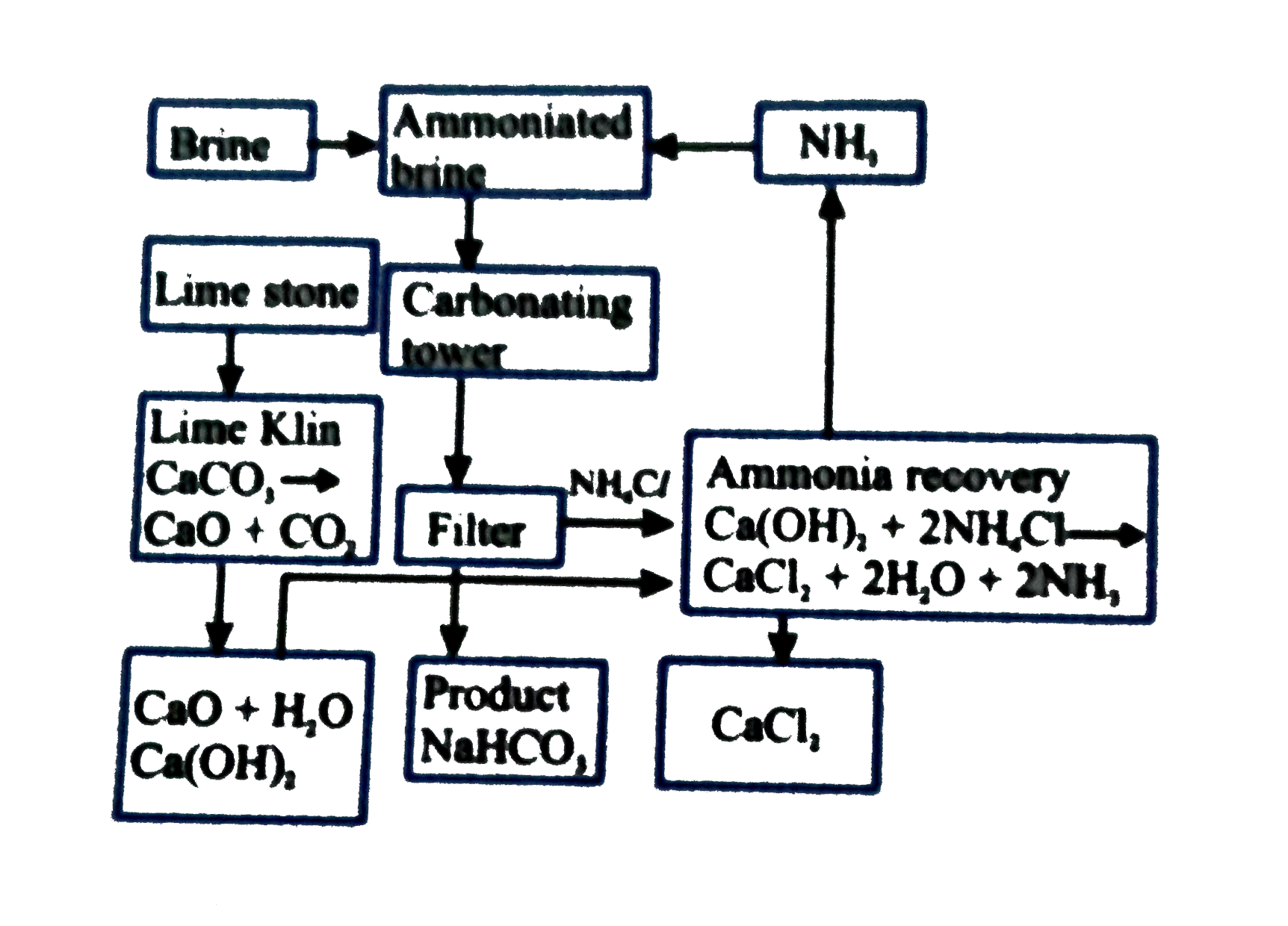
The above process is known asAOstwald processBSolvay processCContact processDNone of theseSodium carbonate is generally prepared by a process called ammonia soda process as follows :
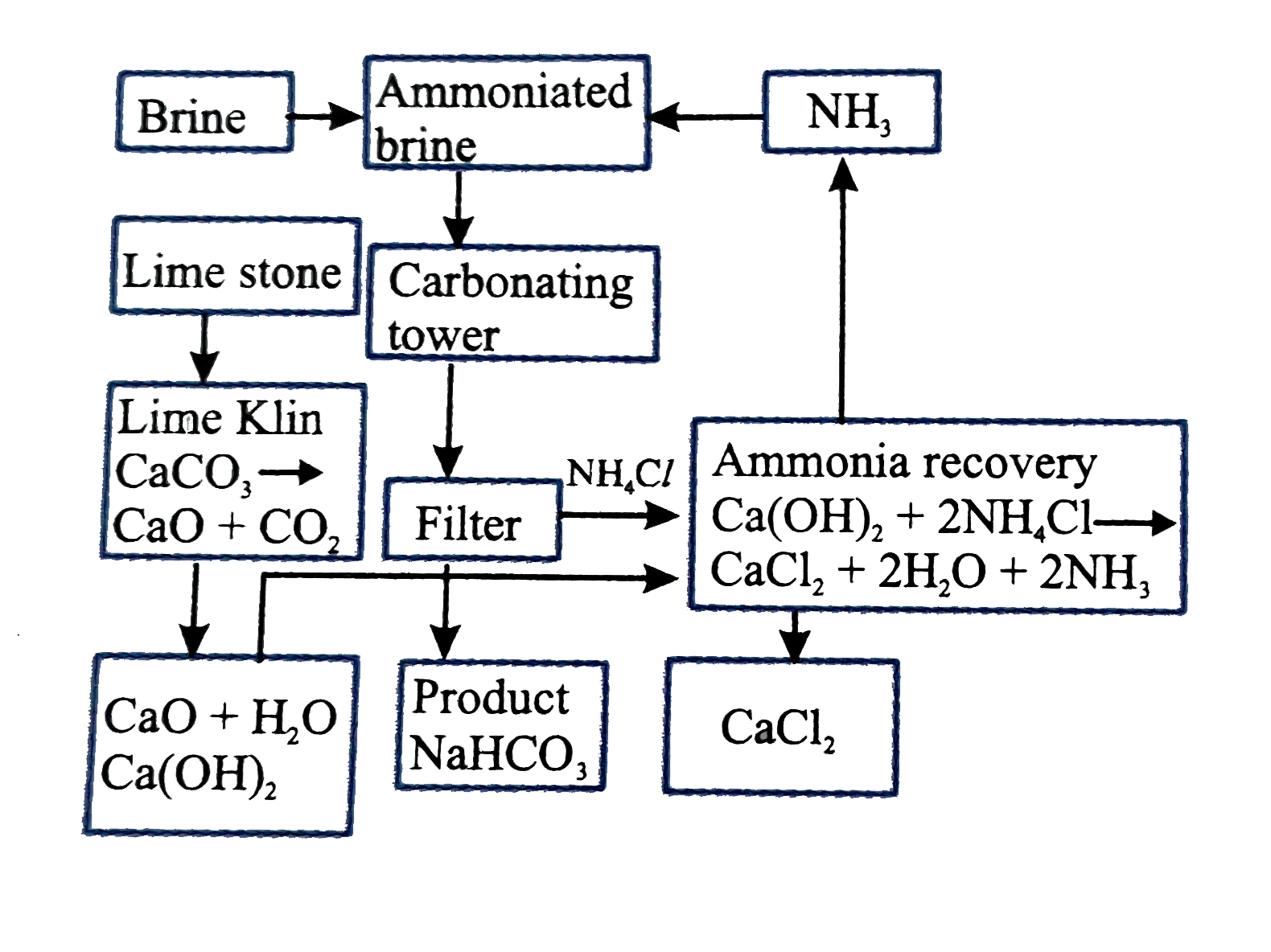
Which of the following is by-product in the above process?ANH4ClBCaCl2CNH4OHDCaOIn Solvay ammonia process, sodium bicarbonate is precipitated due to
ANaHCO3BNa2CO3.10H2OCNa2CO3DAll of these
k2CO3 cannot be prepared by solvay process because
View Solutionk2CO3 cannot be prepared by solvay process because
View Solutionk2CO3 cannot be prepared by solvay process because
View Solutionk2CO3 cannot be prepared by solvay process because
View SolutionSodium carbonate is manufactured by Solvay process. The products that are recycled are
View Solution

