If dissociation constant of ammonia is 10−5, its pKb and pK4 value respectively are
A
5 and 9
B
9 and 5
C
7 and 7
D
4 and 10
Video Solution
Text Solution
The correct Answer is:A
Kb of NH3 is 10−5 (given)
∴pKb=5 and pKa=14−5=9
Hence, pKb and pKa values are 5 and 9 respectively.
|
Similar Questions
Knowledge Check
Maximum pKb value is of
A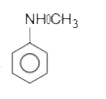 B(CH3CH2)2NHC(CH3)2NHD
B(CH3CH2)2NHC(CH3)2NHD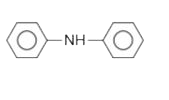
Maximum pKb value is of
A(CH3)2NHB(CH3CH2)2NHC D
D
The degree of dissociation of acetic acid in a 0.1 M solution is 1.0×10−2. The pKa of acetic acid value.
A3B4C5D6
If ionisation constant of an acid (HA) at equilibrium is 1.0×10−8 then calculate the value of pKaandpKb (for its conjugate base).
View SolutionIf ionisation constant of an acid (HA) at equlibrium is 1.0×10−8 then colculte the value of pKaandpKb (for its conjugate base)
View SolutionCalculate the degree of dissociation (ionization) of 0.05 M acetic acid if its pKa is 4.74.
View SolutionThe base with lowest pKa value is
View SolutionCorrect order of pKa value of
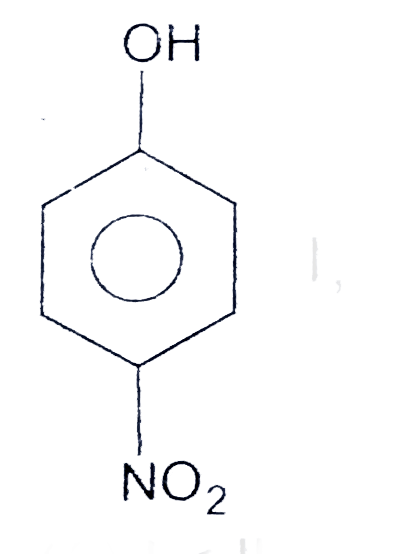
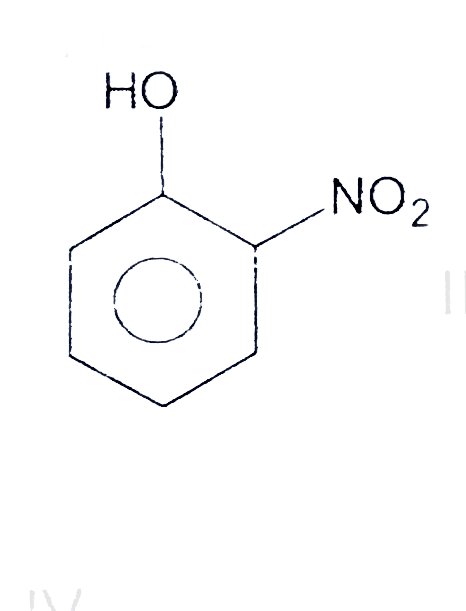
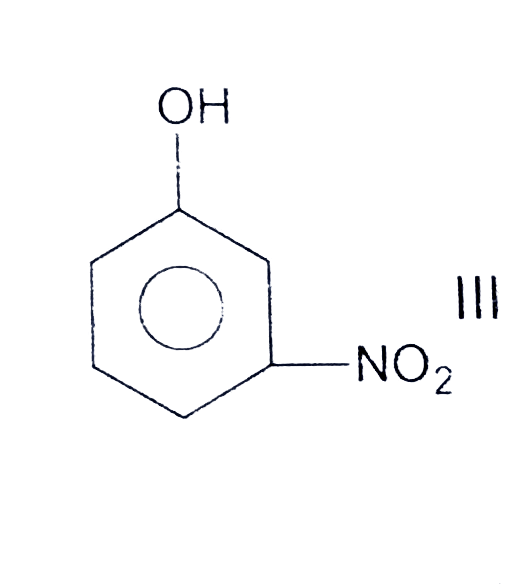
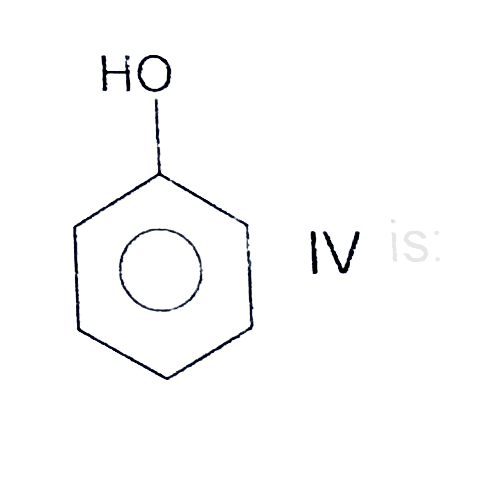
View Solution

