The types of the reactions for these are
I. 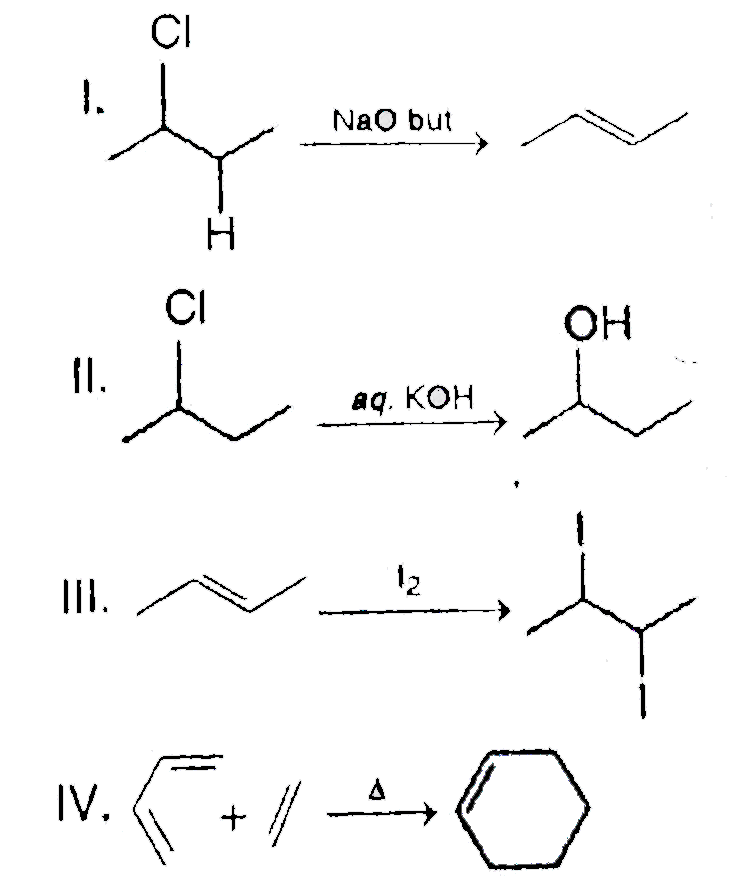
A
elimination, substitution, addition, addition
B
addition, elimination, addition, substitution
C
elimination, addition, substitution, addition
D
substitution, elimination, addition, addition
Video Solution
Text Solution
The correct Answer is:A
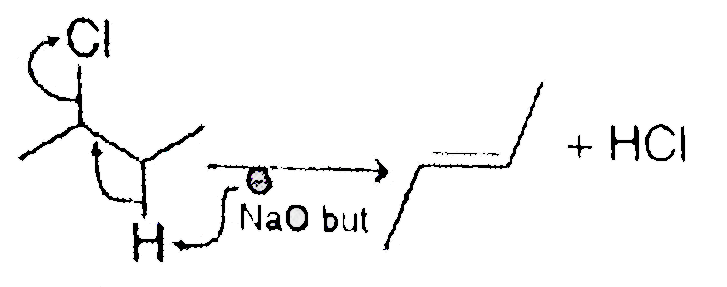
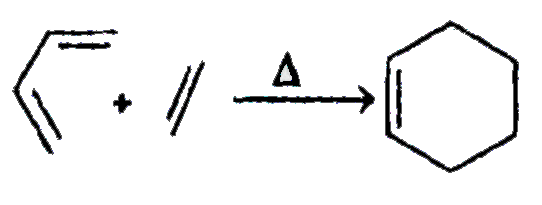
I. Elimination reaction
II. Substitution reaction
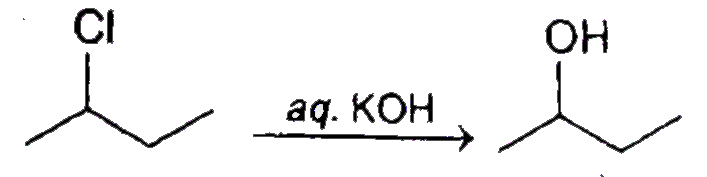
Here, CL replaces by OH.
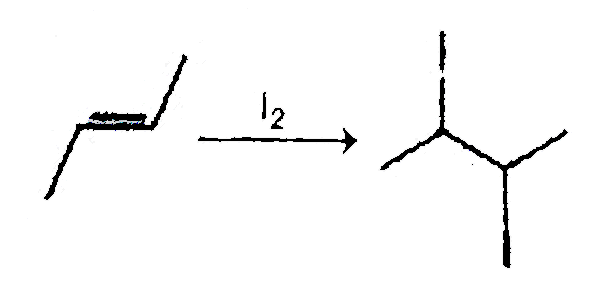
III. Addition reaction
IV. Addition reaction
|
Topper's Solved these Questions
- HYDROCARBONSBook:BITSAT GUIDEChapter:HYDROCARBONSExercise:BITSAT Archives
Explore 19 Videos
- HALOGEN DERIVATIVES OF HYDROCARBONBook:BITSAT GUIDEChapter:HALOGEN DERIVATIVES OF HYDROCARBONExercise:BITST Archives
Explore 10 Videos
- METALLURGYBook:BITSAT GUIDEChapter:METALLURGYExercise:Bitsat Archives
Explore 6 Videos
Similar Questions
Knowledge Check
Type of reactions shown by alkynes are −
AElectrophilic addition reactionsBNucleophilic addition reactionsCSubstitution reactionDAll of the aboveMatch the reactions in Column I with nature of the reactions/type of the products in Column II
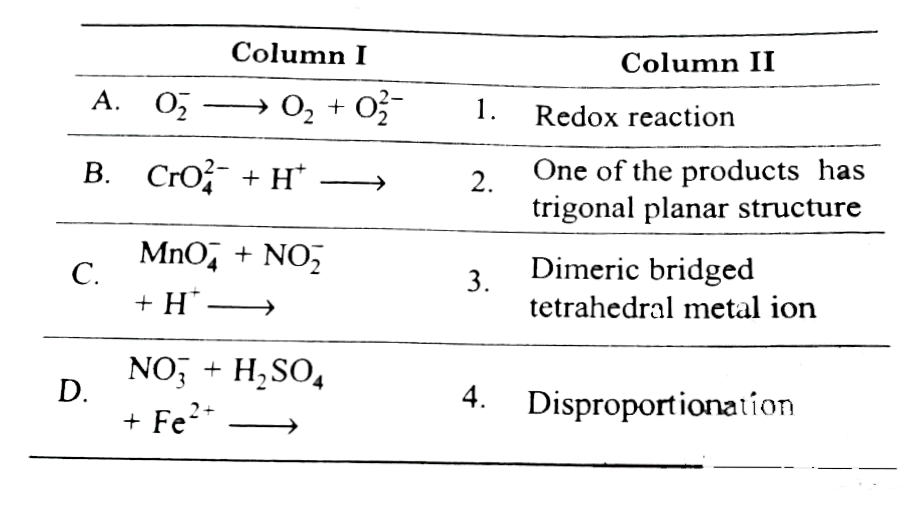 AABCD21,434BABCD1,431,21CABCD2314DABCD342,31
AABCD21,434BABCD1,431,21CABCD2314DABCD342,31Match the reactions in Column I with nature of the reactions / type of the products in Column II.
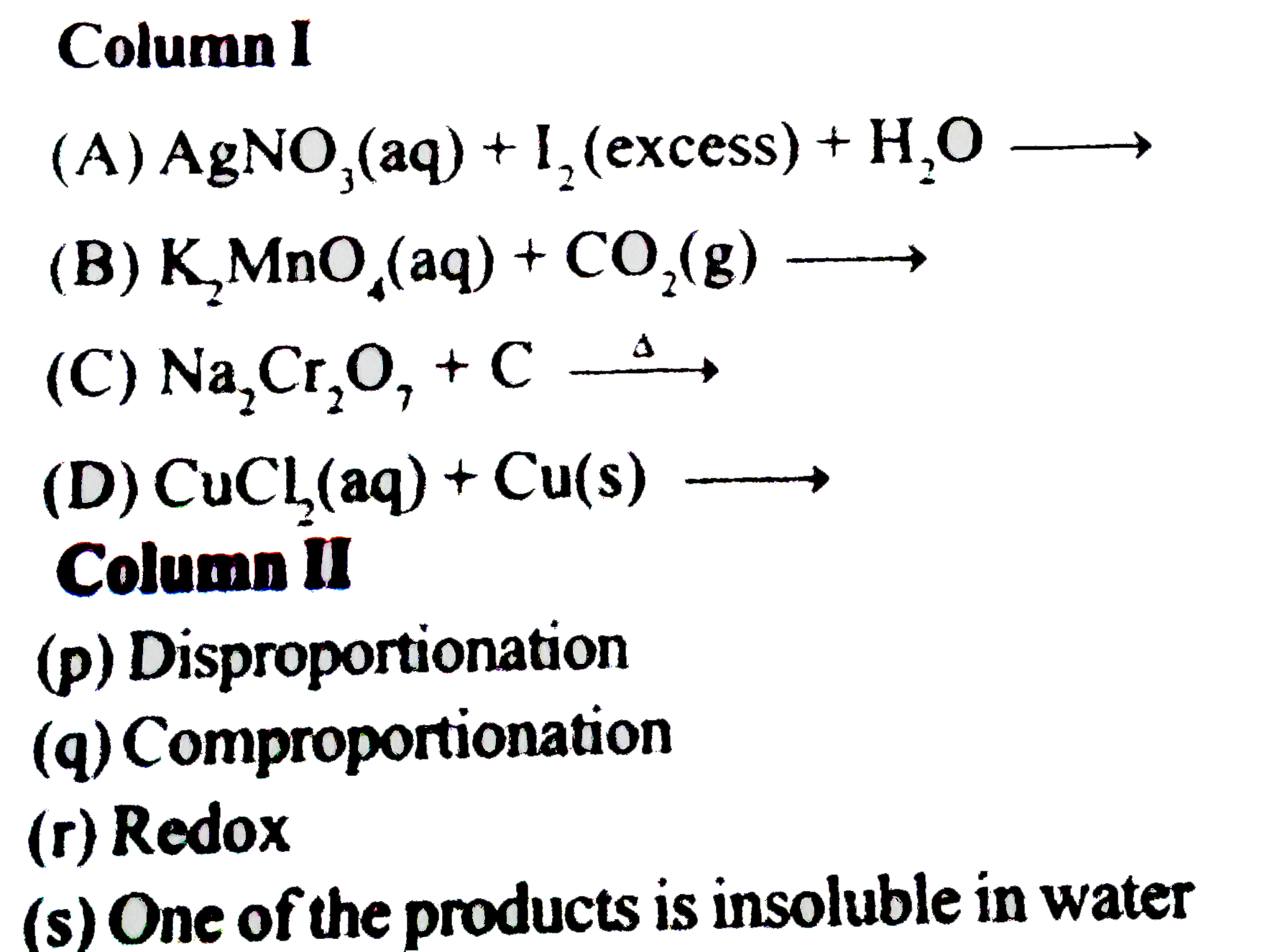 AAgNO3(aq)+I2(excess)+H2O→ (p)DisproportionationBK2MnO4(aq)+CO2(g)→ (p)ComproportionationCNa2Cr2O7+CΔ−−→ (r)Redox reactionDCuCl2(aq)+Cu(s)→ (s)One of the products is insoluble in water
AAgNO3(aq)+I2(excess)+H2O→ (p)DisproportionationBK2MnO4(aq)+CO2(g)→ (p)ComproportionationCNa2Cr2O7+CΔ−−→ (r)Redox reactionDCuCl2(aq)+Cu(s)→ (s)One of the products is insoluble in water
Name of type of chemical reaction represented by the following equation :
(i)CaO+H2O→Ca(OH)2
View SolutionMatch the reactions given in column I with the types of reactions given in column II.
 .
.
View SolutionMatch the reactions in Column I with nature of the reactions / type of the products in Column II.
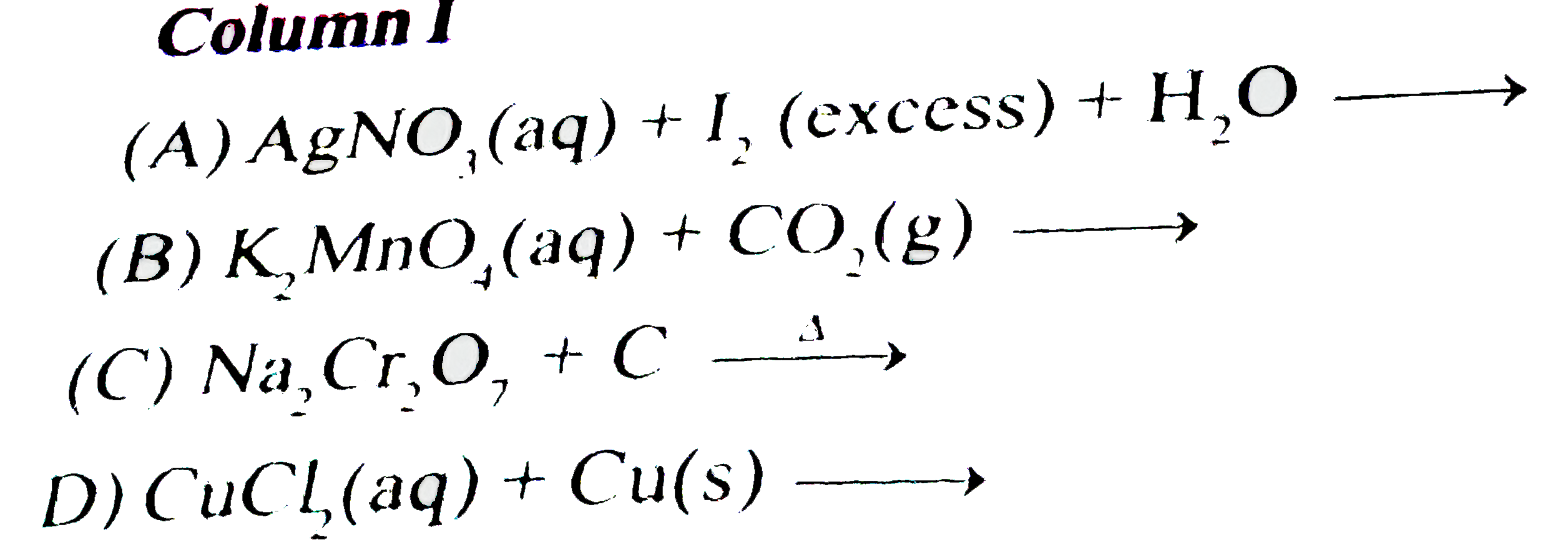
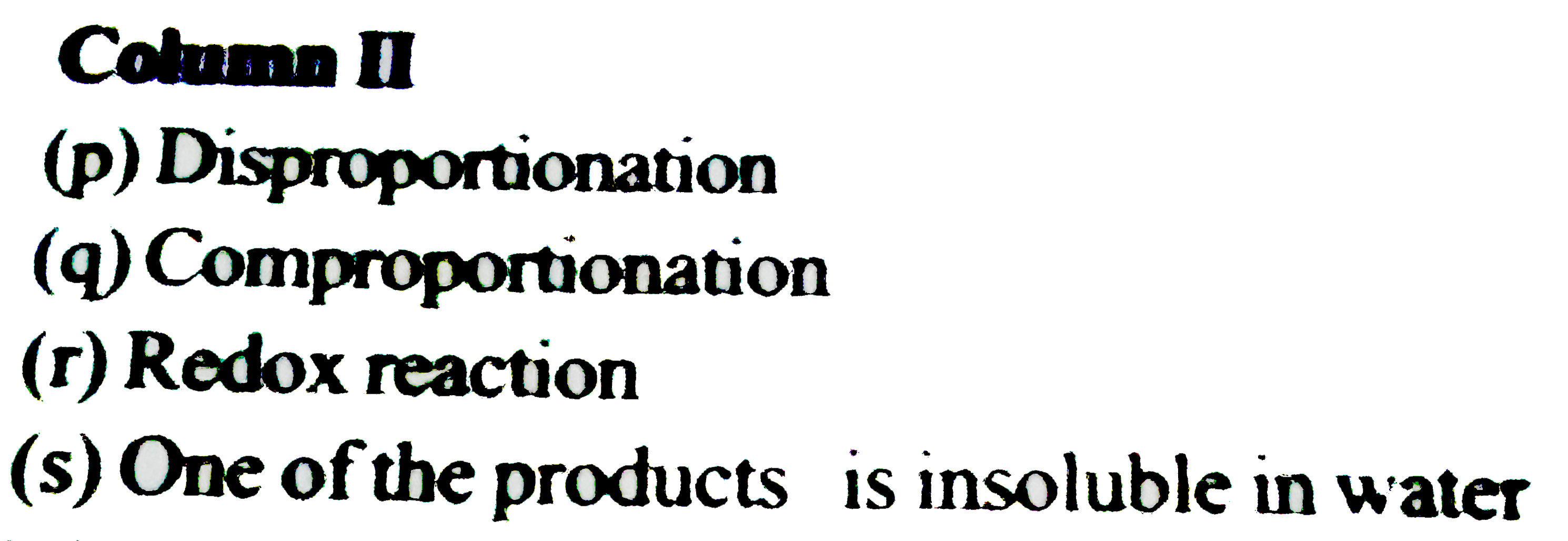
View SolutionMatch the reactions in Column I with nature of the reactions / type of the products in Column II.
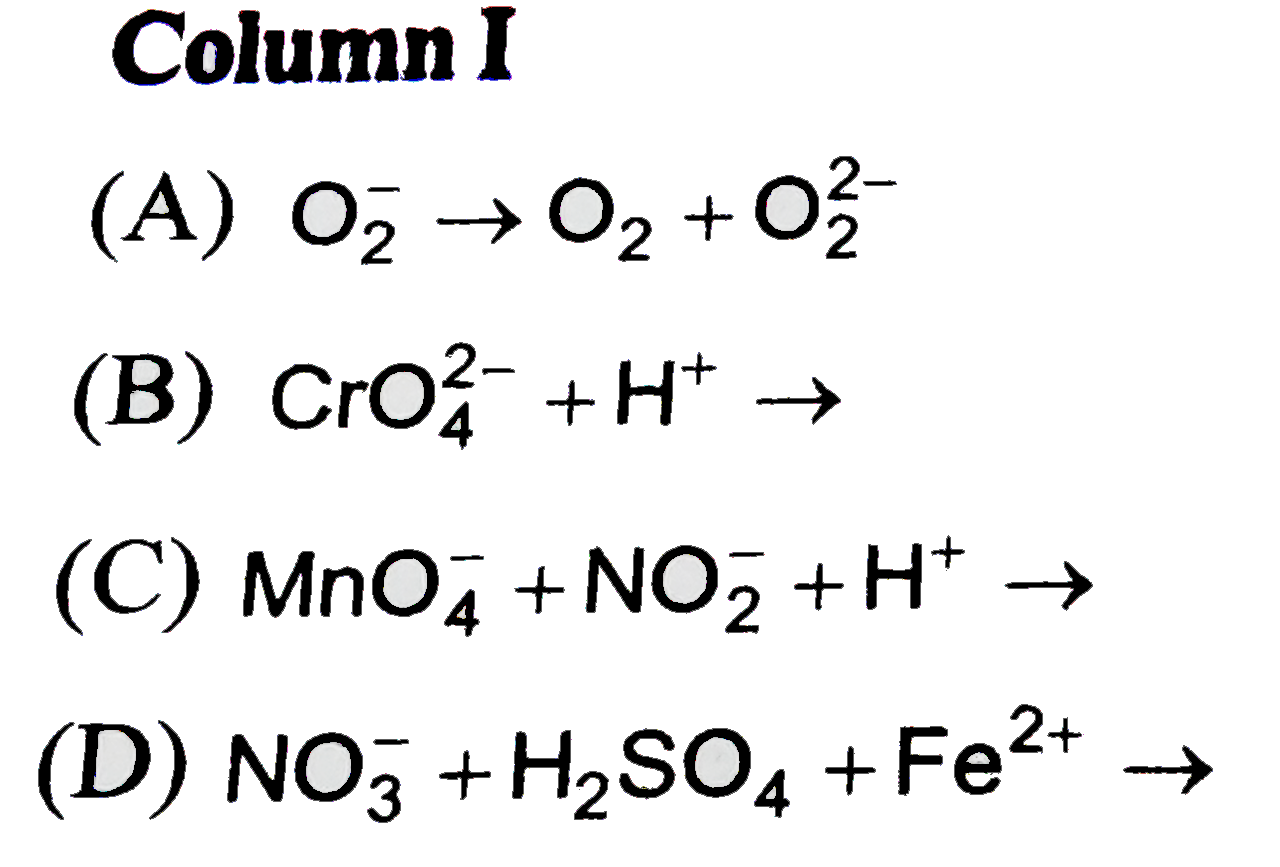
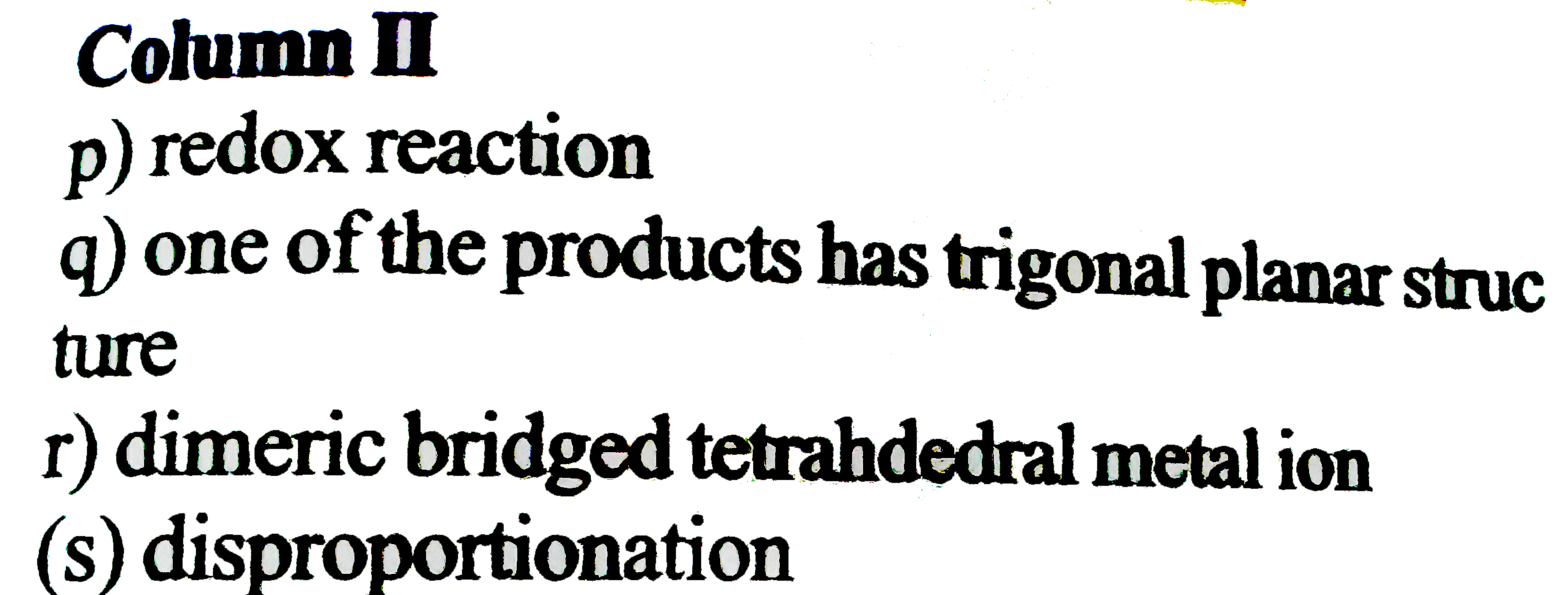
View SolutionMatch the reactions in Column I with nature of the reactions / type of the products in Column II.

View Solution
