Acetic acid reacts separately with the following alcohols. The rate of esterfication is highest for
A
CH3OH
B
C2H5OH
C
(CH3)2CHOH
D
(CH3)3COH
Video Solution
Text Solution
The correct Answer is:a
As the hydrocarbon part (R) of the alcohol increases the tetraehderal intermediate (l) formed during esterification becomes more and more crowded and hence, the rate of esterification decreases accordingly. Thus, the smallest alcohol, i.e. CH3OH reacts at the fastest rate.
CH3−O∣∣C−OH→CH3−+OH∣∣C−OHROH−−→
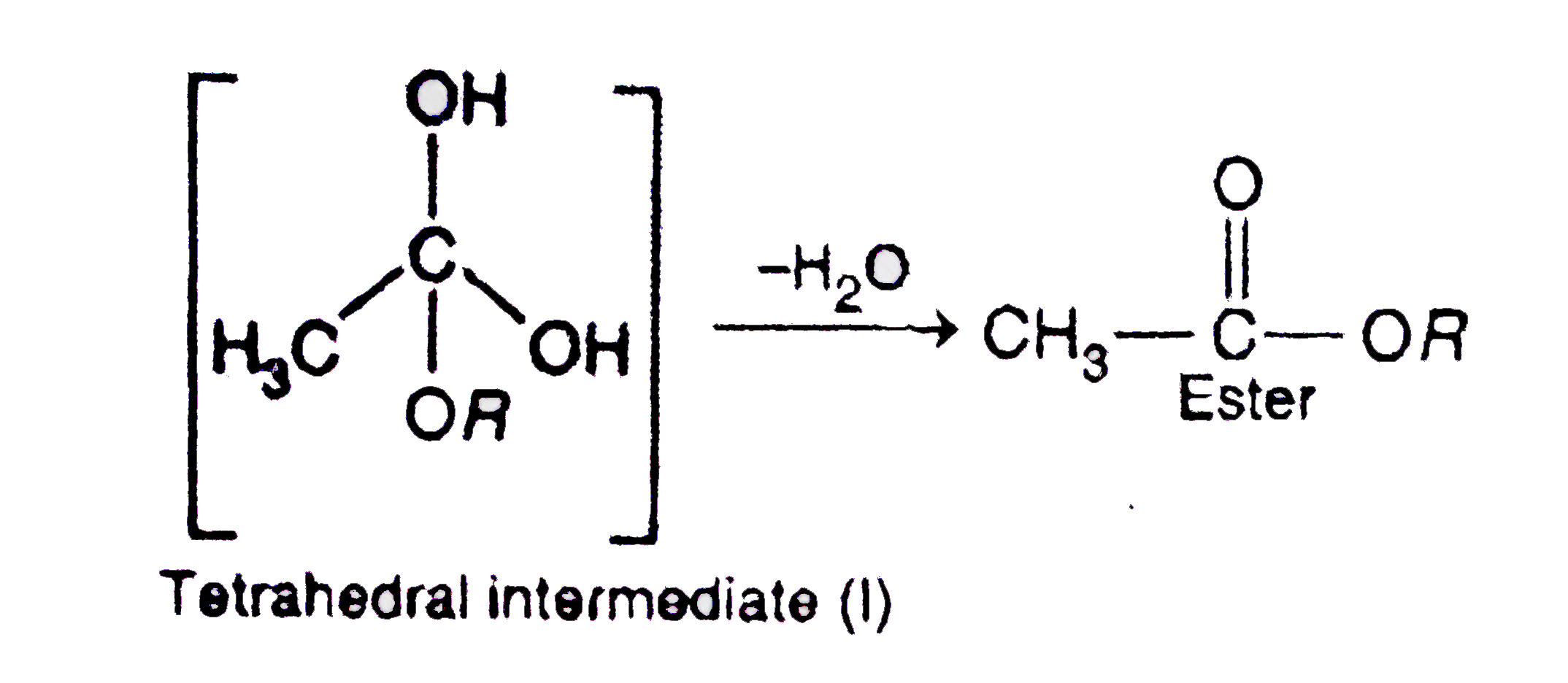
|
Similar Questions
Knowledge Check
Acetic acid reacts separately with the following alcohols. The rate of esterification is higher for :
ACH3OHBC2H5OHC(CH3)2CHOHD(CH3)3COHRate of esterification is highest for the acid
ACH3(CH2)2COOHB(CH3)2CHCOOHCCH3COOHDCl(CH2)4COOHAcetic acid reacts with
ANH3BalcoholCNaHCO3DNaOH
Acetic acid reacts with PCl5 to form
View SolutionAcetic acid react with PCl5 gives ,
View SolutionWhich of the following will react at the highest rate ?
View SolutionWhich of the following will react at the highest rate ?
View SolutionWhich of the following will react at the highest rate ?
View Solution
