Regarding H3PO3, its structur is as follows
HO−O||P∣H−H
Its basicity is one. Its salts are known as hyphophosphites
In the above structure the no. of Pπ−dπ bonds
Reducing agent
Dehydrating agent
Drying agent
Pickling agent
The correct Answer is:A, D
Step by step video, text & image solution for Regarding H_(3)PO_(3), its structur is as follows HO-underset(underset(H)(|))overset(overset(O)("||"))(P)-H Its basicity is one. Its salts are known as hyphophosphites In the above structure the no. of P pi-dpi bonds by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- 15TH GROUP ELEMENTSBOOK - NARAYNACHAPTER - 15TH GROUP ELEMENTSEXERCISE - Level-I (H.W)
36 Videos
- 15TH GROUP ELEMENTSBOOK - NARAYNACHAPTER - 15TH GROUP ELEMENTSEXERCISE - Level-II (H.W)
41 Videos
- 15TH GROUP ELEMENTSBOOK - NARAYNACHAPTER - 15TH GROUP ELEMENTSEXERCISE - Level-III
45 Videos
- ELECTRO CHEMISTRYBOOK - NARAYNACHAPTER - ELECTRO CHEMISTRYEXERCISE - SUBJECTIVE QUESTIONS
48 Videos
- 16 TH GROUP ELEMENTS BOOK - NARAYNACHAPTER - 16 TH GROUP ELEMENTS EXERCISE - EXERCISE - 4 NCERT EXAMPLERS/H.O.T.S.
18 Videos
Similar Questions
Atomicity of white phosphorus is
View SolutionHow do we get pure N2 gas
View SolutionWhich of the following statements is not true
View SolutionCopper sulphate solution (250ML) was electrolyzed using a platinum anode and a copper cathode. A constant current of 2mA was passed for 16min. It was found that after electrolysis the absorbance of the solution was reducted to 50% of its original value . Calculate the concentration of copper sulphate in the solution to begin with.
View SolutionThe sulphur molecule (S8) can be represented as a
View SolutionIn structure Cl−O∣∣S∣∣O−Cl and
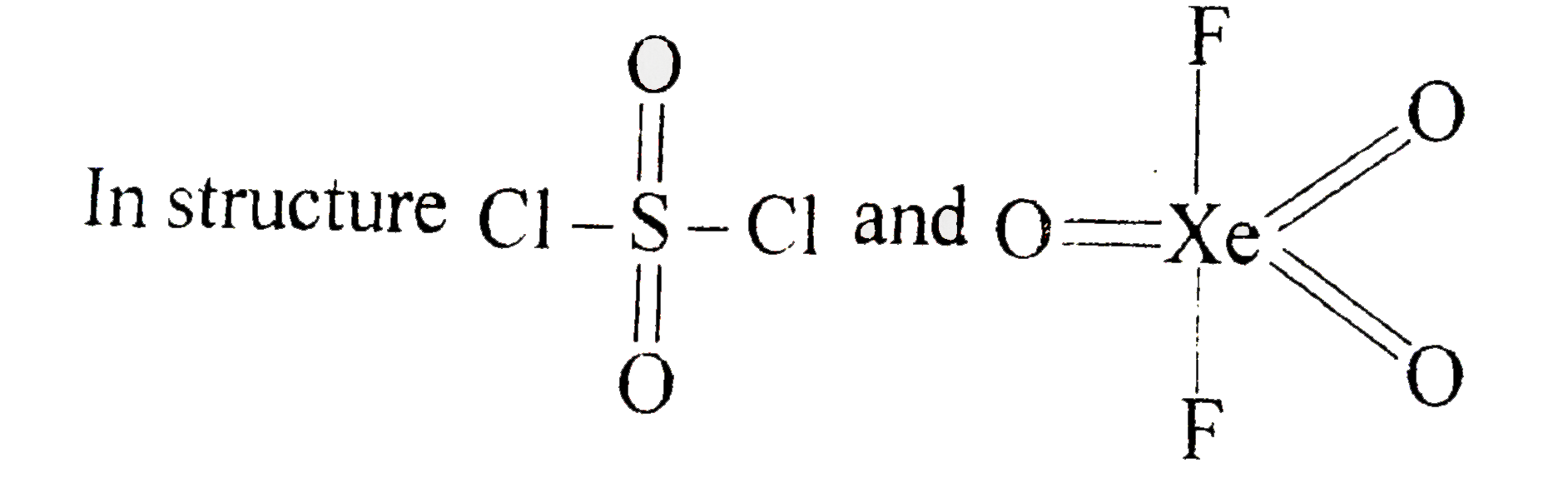
Number of pπ−dπ bonds present in it respectively
View SolutionRegarding H_(3)PO_(3) , its structur is as follows HO-underset(underset(H)(|))overset(overset(O)("||"))(P)-H Its basicity is one. Its salts are known as hyphophosphites In the above structure the no. of P pi-dpi bonds
View SolutionRegarding H3PO3, its structur is as follows
HO−O||P∣H−H
Its basicity is one. Its salts are known as hyphophosphites
In the above structure the no. of Pπ−dπ bonds
View SolutionRegarding H3PO3, its structur is as follows
HO−O||P∣H−H
Its basicity is one. Its salts are known as hyphophosphites
In the above structure the no. of Pπ−dπ bonds
View SolutionIdentify product (A) and write its structure. Ph - underset(Ph)underset(|)overset(Ph)overset(|)(CH) underset(AlCl_(3))overset(H^(+))rarr
View SolutionThe structural formula of an ester is:
H−H∣C∣H−O∣∣C−O−H∣C∣H−H∣C∣H−H
Write the structural formula of the corresponding alcohol and acid.
View SolutionAssertion : The resonance structure of
H−H∣C∣H−O−H is H−H∣C∣H=+O−H
Reason : Methanol cannot be represent by a resonance structure since the carbon atom has five bonds.
View Solutionनिचे दी गई संरचनाओं (1 तथा 2 ) द्वारा H3PO3 को प्रदर्शित किया जा सकता है । क्या ये दो संरचनाएँ H3PO3 के अनुनाद संकर के विहित ( केनॉनीकल ) रूप माने जा सकते है ? यदि नहीं तो इसका कारण बताइए । HH:⋅⋅O⋅⋅:⋅⋅P⋅⋅:⋅⋅O⋅⋅:H :O⋅⋅: (1) H:⋅⋅O⋅⋅:⋅⋅P⋅⋅:⋅⋅O⋅⋅:H :O⋅⋅: H (2)
View SolutionThe skeleton structure for acetaldehyde (CH_(3)CHO) is : H" "overset(H)underset(H)C" "underset(H)C" "O . Draw its Lewis structure.
View Solutionप्रक्कथन : H−H∣C∣H−O−H की अनुनादी संरचना
H−H∣C∣H=+O−H है।
कारण : मेथेनॉल को अनुनादी संरचना द्वारा प्रदर्शित नहीं किया जा सकता है, चूँकि कार्बन परमाणु में 5 बन्ध होते हैं।
View Solution

