The number of π bonds in perxenate ion is :
Video Solution
Text Solution
The correct Answer is:2
|
Answer
Step by step video, text & image solution for The number of pi bonds in perxenate ion is : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The number of peroxide bonds in perxenate ion [XeO6]4− is
A0B2C3D1The number of peroxide bonds in perxenate ion [XeO6]4− is
A0B2C3D1The nature of π-bonds in perchlorate ion
AO−Cl(dπ−pπ)BO−Cl(pπ−dπ)CO−Cl(dπ−dπ)DNone of these.


 The number of pi bonds in the final product is
The number of pi bonds in the final product is 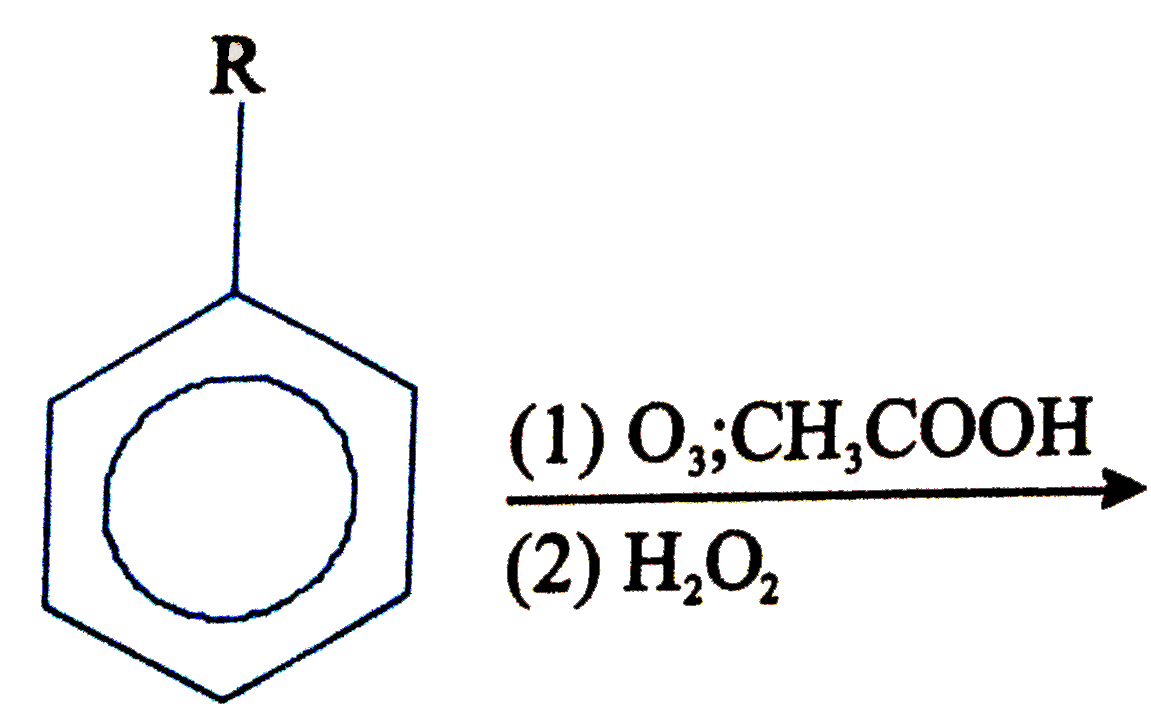 Products. The total number of
Products. The total number of 