The ratio of magnetic moment of Fe(III) and Co(II) is :
A
√5:√7
B
√35:√15
C
7:3
D
√24:√15
Video Solution
Text Solution
The correct Answer is:B
Fe3+→3d5
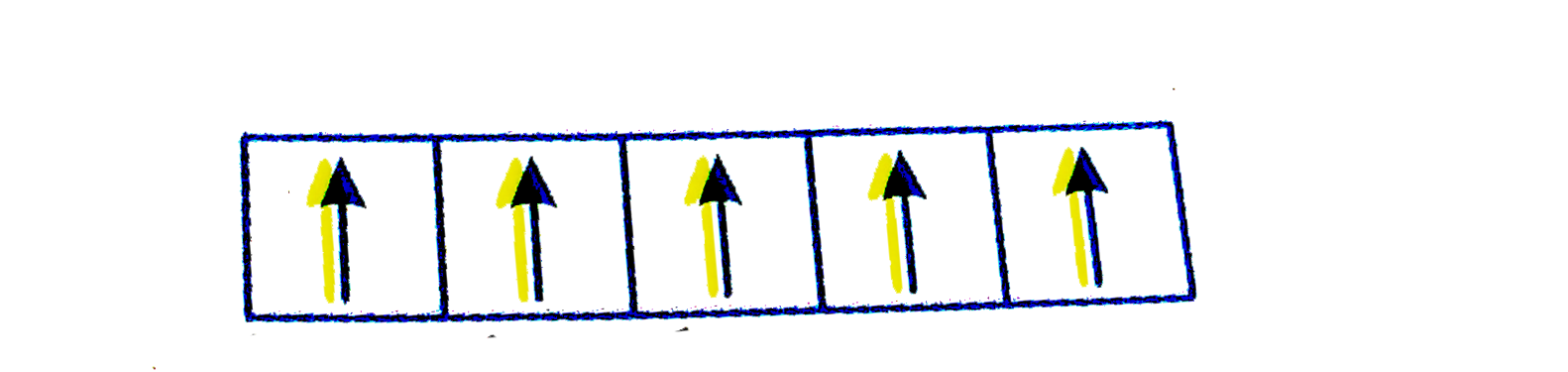 5 unpaired electrons
5 unpaired electrons
Co2+→3d7
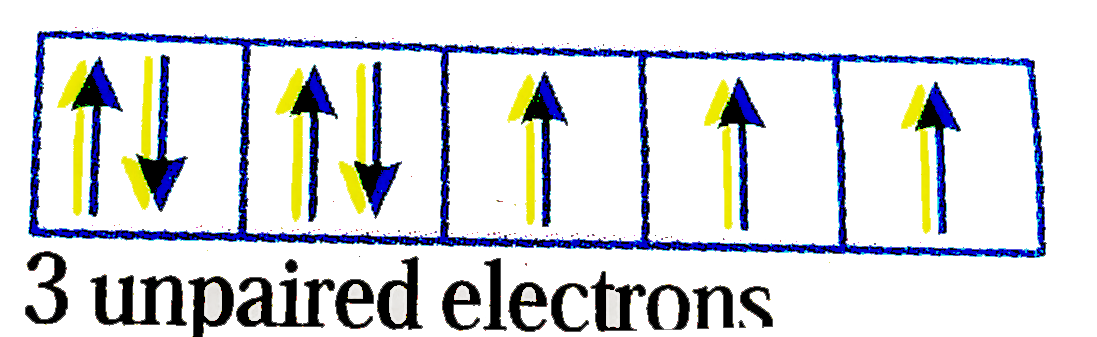
3 unpaired electrons
|
Similar Questions
Knowledge Check
The ratio of magnetic moments of Fe(III) and Co(II) is :
A√5:√7B√35:√15C7:3D√24:√15The ratio of magnetic moments of Fe(III) and Co(II) is:
A√5:√7B√35:√15C7:3D√24:√15Ratio of magnetic moment of the ions Co2+andCr3+ is :
A√15B1C3D2
The spin only magnetic moment of Fe3+ ion (inBM) is approximately
View SolutionThe spin only magnetic moment of Fe2+ ion (in BM) is approximately
View SolutionThe spin only magnetic moment of Fe3+ ion (in BM) is approximately :
View SolutionThe spin only magnetic moment of Fe3+ ion (inBM) is approximately
View SolutionWhen an electron revolves around the nucleus, then the ratio of magnetic moment to angular momentum is
View Solution
