The compounds that undergo hydrolysis readily is/are
A
TiCl4
B
VCl5
C
FeCl3
D
CaCl2
Video Solution
Text Solution
The correct Answer is:A, B, C
Transition metal compounds that are covalent undergo hydrolysis. CaCl2 is ionic and no hydrolysis.
|
Similar Questions
Knowledge Check
The compound that undergoes dehydration most readily is
AEthyl alcoholB2-methylpropan-2-olC3-methyl-2-butanolDPropyl alcoholAmong the following , the compound that undergoes nitration readily is
ABenzoic acidBTolueneCPhenolDNitrobenzeneWhich one of the following compounds undergoes hydrolysis most readily by aq. NaOH to furnish corresponding phenol ?
A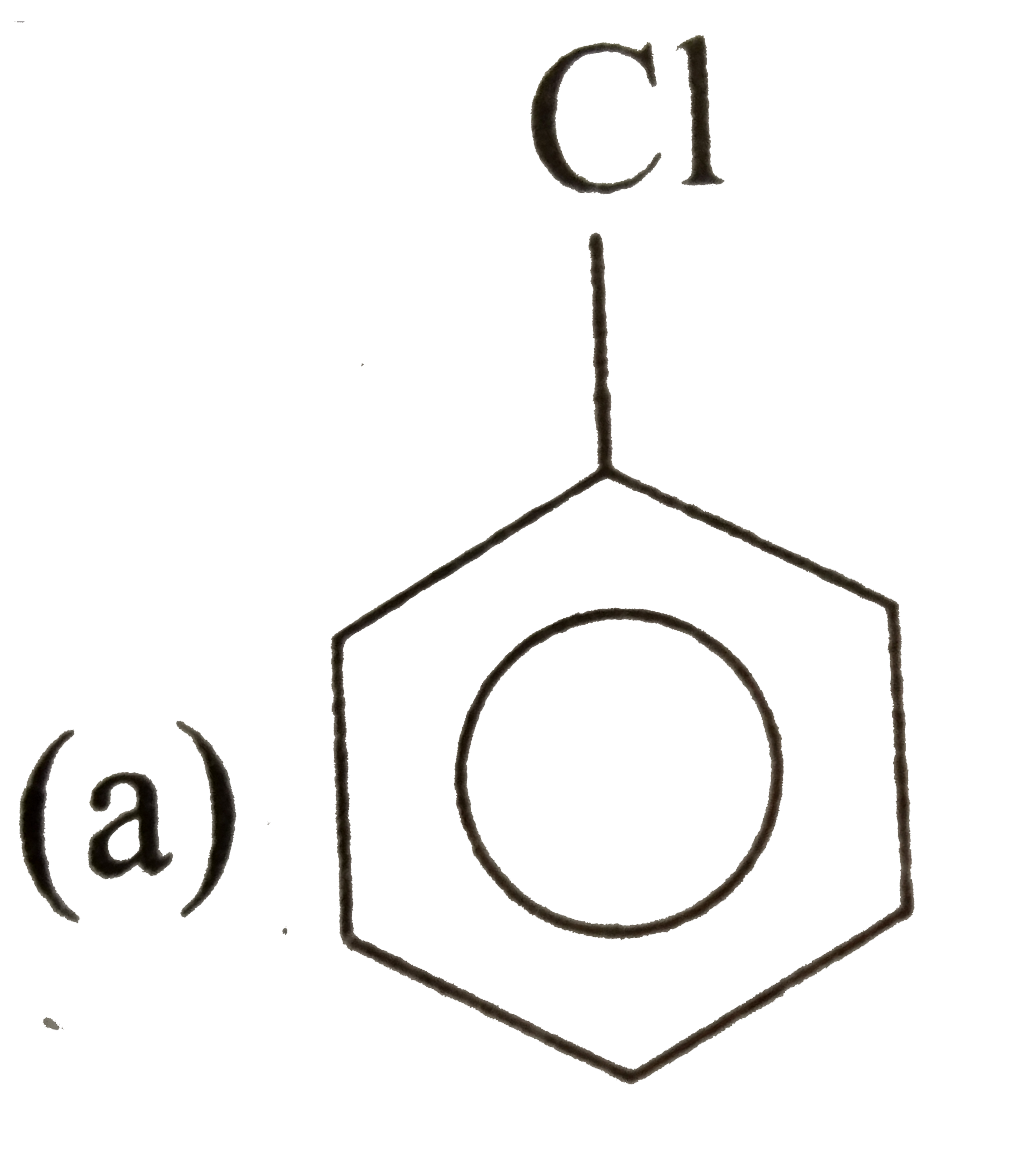 B
B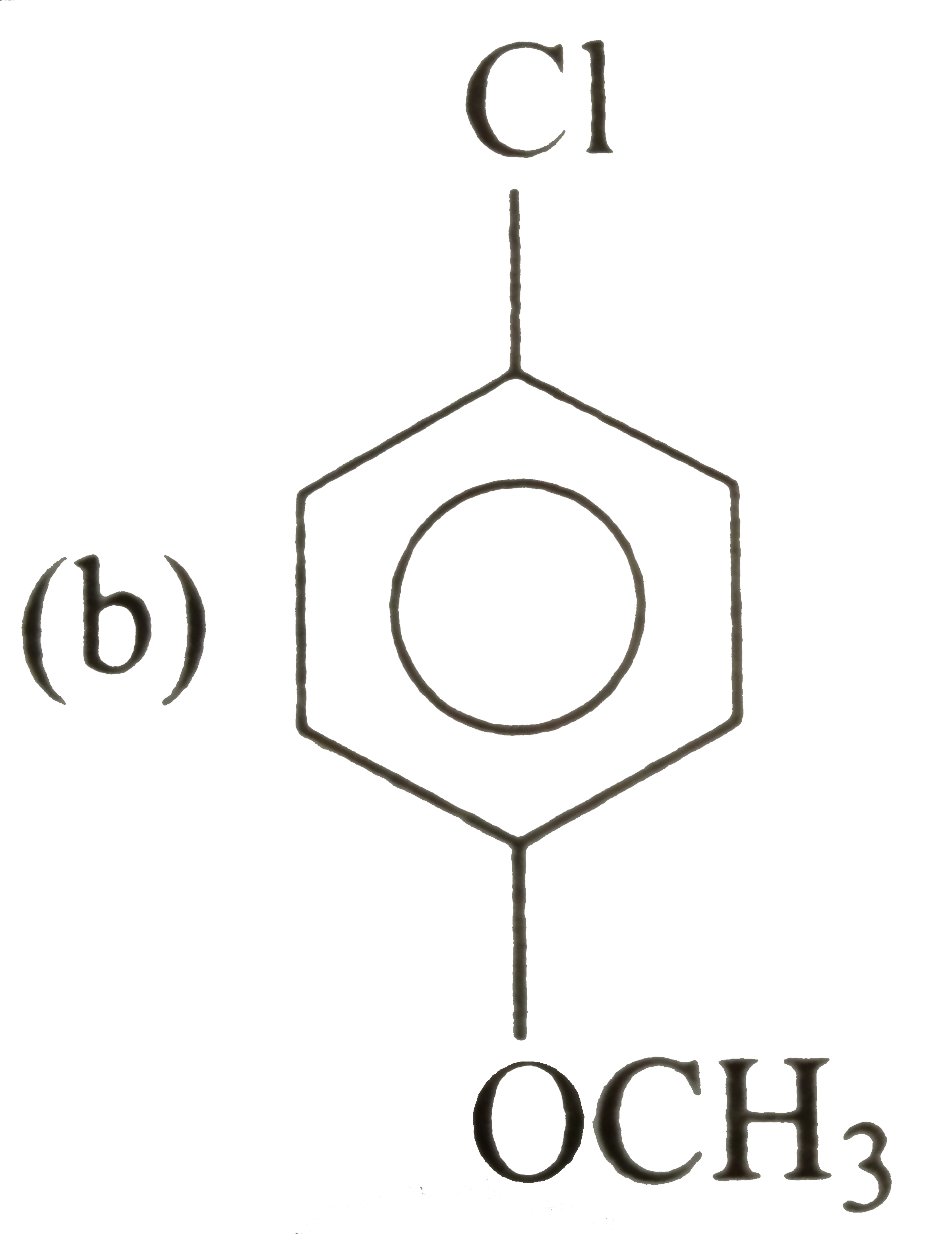 C
C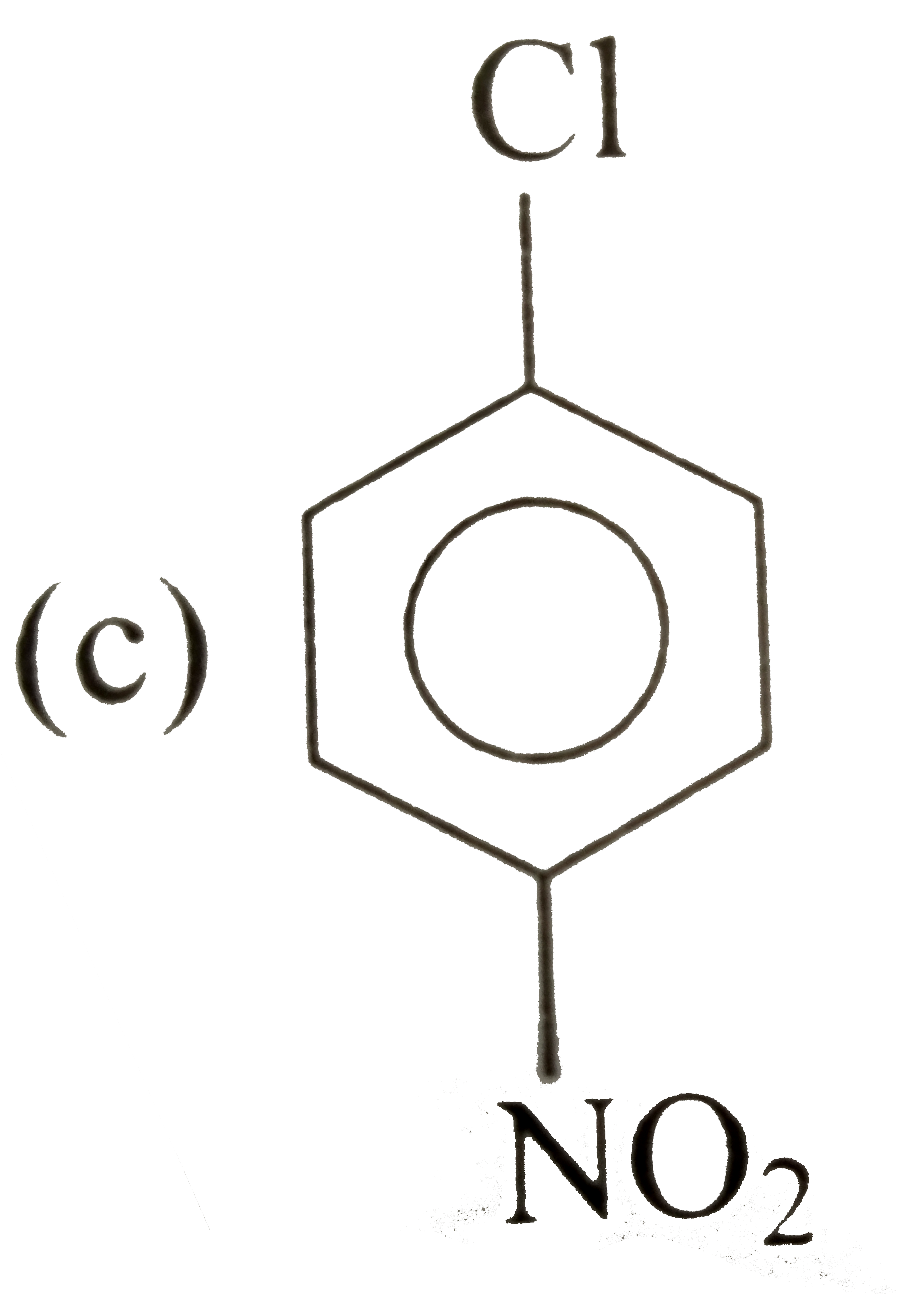 D
D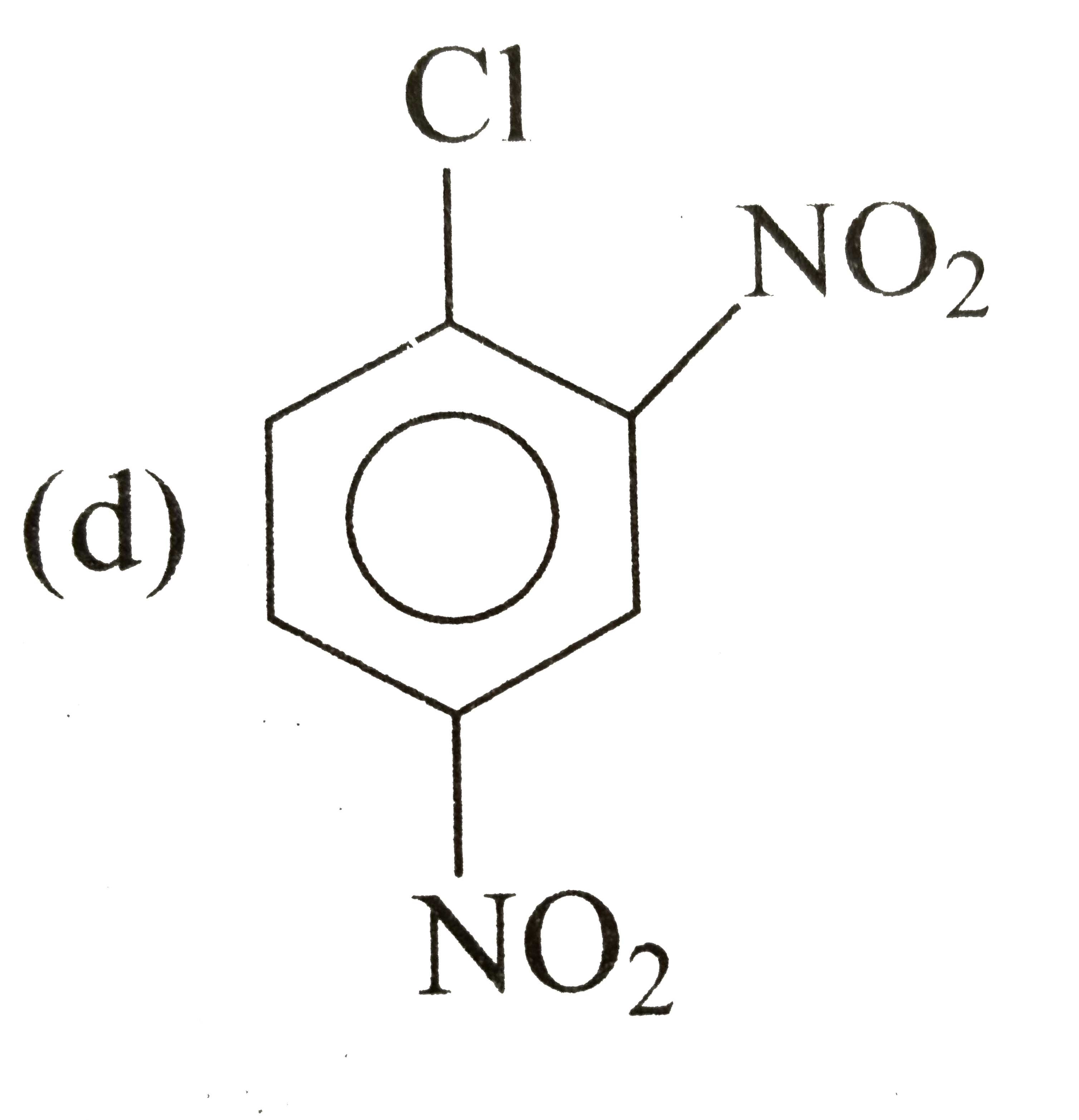
The compound that does not undergo hydrolysis by SN1 mechanism is :
View SolutionWhich compound does not undergo hydrolysis?
View SolutionThe compound which does not undergo hydrolysis at room temperature is :
View SolutionWhich of the following compound undergo hydrolysis most easily :
View SolutionThe compound which does not undergo hydrolysis by SN1 mechanism is:
View Solution

