A chiral C5H10O alcohol is reduced by catalytic hydrogenation to an achiral C5H12O alcohol. The original alcohol is oxidized by activated MnO2 to an achiral carbonyl compound (C5H8O) Which of the following might be the chiral alcohol ?
1- peten-3-ol
4- peten-2-ol
3-methyl-2-buten-1-ol
2-methyl-2-buten-1-ol
The correct Answer is:A
Similar Questions
Compound X (C5H10O) is a chiral alcohol. It is catalytically hydrogenated to an achiral alcohol Y (C5H12O) and oxidized by activated MnO2 to an achiral carbonyl compound Z(C5H8O). Compound X is
View SolutionA C7H14O optically active alcohol is oxidized by jones reagent to an optically inactive (achiral) ketone. Which of the following compounds meets these facts ?
View Solution
Knowledge Check
Compound X (C5H10O) is a chiral alcohol. It is catalytically hydrogenated to an achiral alcohol Y (C5H12O) and oxidized by activated MnO2 to an achiral carbonyl compound Z(C5H8O). Compound X is
A1-penten-3-olB1-penten-2-olC3-methyl-2-buten-1-olD2-methyl-2-butene-1-olA chiral C7H16O2 diol is oxidized by PCC in CH2Cl2 to an achiral C7H12O2 compound. Which of the following would satisfy these facts ?
A B
B C
C D
D
A chiral C7H16O2 diol is oxidized by PCC in CH2Cl2 to an achiral C7H12O2 compound. Which of the following would satisfy these facts?
A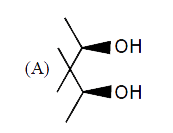 B
B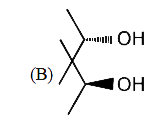 C
C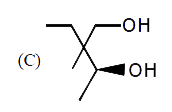 D
D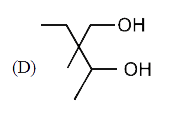
AC6H14O chiral alcohol is converted to a bromide by treatment with PBr3. Reaction of this bromide, first with Mg in ether , followed by quenching in 0.1NHCl produces an achiral C6H14 hydrocarbon. Which of the following is the original alcohol?
View SolutionAn alcohol of formula C9H12O reacts with Na2Cr2O7 to form a compound having formula C9H10O. The original alcohol might be :
View SolutionAn alcohol of formula C9H12O reacts with Na2Cr2O7 to form a compound having formula C9H10O. The original alcohol might be
View SolutionAn optical active alcohol of formula C4H10O on oxidation given which of the following compound?
View SolutionA C7H12O2 optically active alcohol is oxidised by jones reagent to an optically inactive ketone. The molecule is.
View Solution

