From the following data, mark the option (s) where ΔH is correctlt written for the given reaction. Given
H+(aq)+OH−(aq)→H2O(l),ΔH=−57.3kJ
ΔHsolution of HA(g) = -70.7kJ/mol
ΔHsolution of BOH(g) = 20kJ/mol
ΔHionization of HA = 15 kJ/mol and BOH is a strong base Reaction ΔHr(kJ/mol)
HA(aq)+BOH(aq)→BA(aq)+H2O−42.3
HA(g)+BOH(g)→BA(aq)+H2O−93.0
HA(g)→H+(aq)+A−(aq)−55.7
B+(aq)+OH−(aq)→BOH(aq)−20.0
The correct Answer is:A, B, C
(a) −57.3−(−15) , (b) −70.7+20+15−57.3
(c) −70.7+15
Topper's Solved these Questions
- THERMODYNAMICSBook:NARAYNAChapter:THERMODYNAMICSExercise:Level-VI
Explore 150 Videos
- THERMODYNAMICSBook:NARAYNAChapter:THERMODYNAMICSExercise:Passages
Explore 13 Videos
- THERMODYNAMICSBook:NARAYNAChapter:THERMODYNAMICSExercise:Level-II (H.W)
Explore 24 Videos
- STRUCTURE OF ATOMBook:NARAYNAChapter:STRUCTURE OF ATOMExercise:EXERCISE - IV EXEMPLAR PROBLEMS
Explore 24 Videos
Similar Questions
A certain mass of gas expanded from ( 1L, 10 atm) to (4 L, 5 atm) against a constant external pressure of 1 atm. If initial temperature of gas is 300 K and the heat capacity of process is 50J/∘C. Then the enthalpy change during the process is :
(1Latm˜–100J)
View SolutionGraph for one mole gas is given below
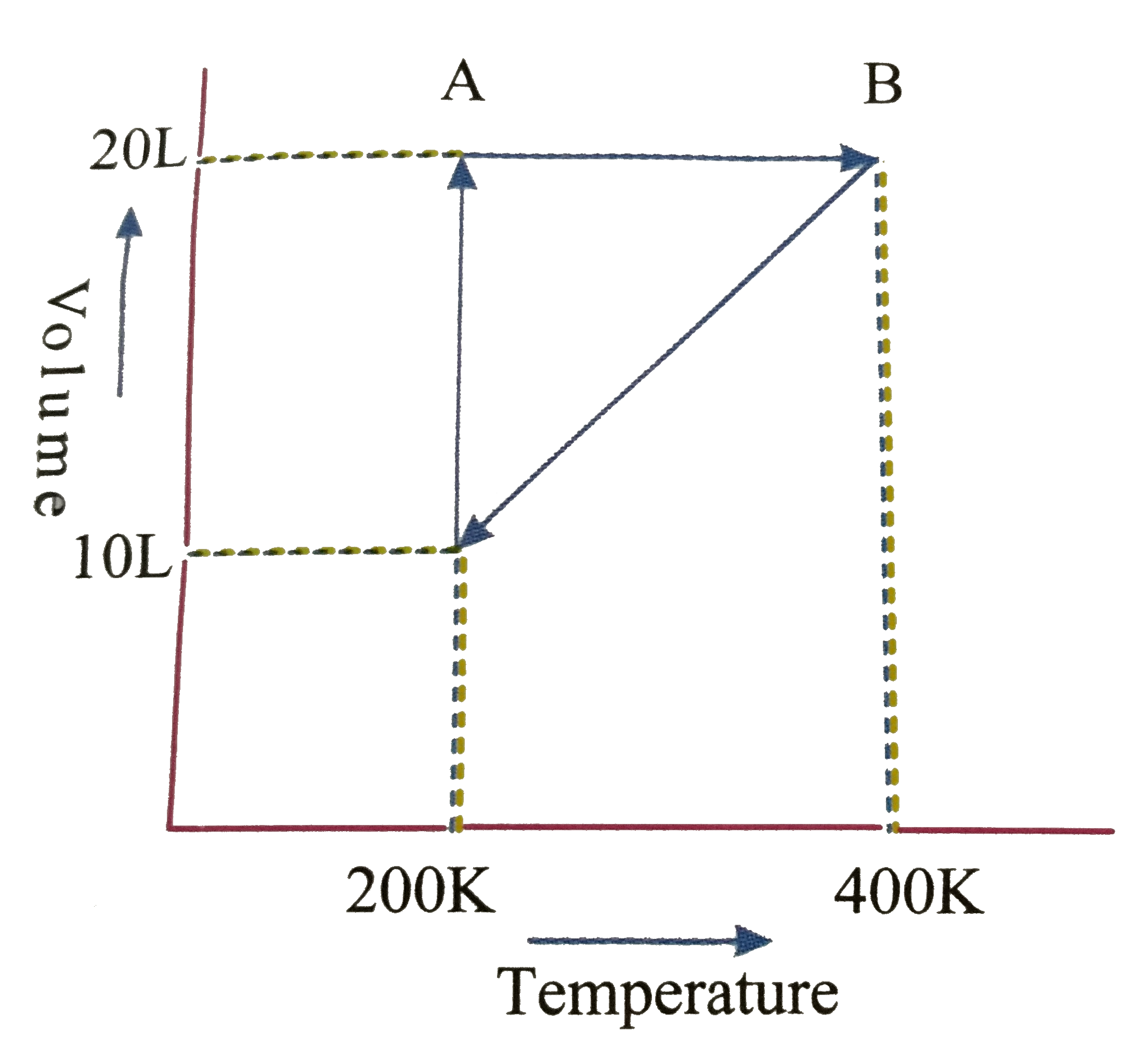
Process, A→B represents
View SolutionA gas is allowed to expand reversibly under adiabatic conditions. What is zero for such a process ?
View SolutionWhich of the following conclusions couldnot be derived from Rutehrford's α-particle scattering experiment?
View SolutionFrom the following date , mark the opation(s) where Delta H is correctly written for the given reaction . Given: H+(aq) +OH-(aq) to H_(2)O(l), DeltaH=-57.3 kJ DeltaH_(solution) HA(g)=-70.7 kJ"mole" DeltaH_(solution) BOH(g)=- 20 kJ"mole" DeltaH_("ionzatoin") of HA=15 kJ//"mole" and BOH is a strong base.
View SolutionDiborane is a potential rocket fuel which undergoes combustion according to the reaction, B_(2)H_(6) (g) + 3O_(2) (g) rarr B_(2) O_(3) (s) + 3H_(2) O (g) From the following data, calculate the enthalpy change for the combustion of diborane: (i) 2 B (s) + ((3)/(2)) O_(2) (g) rarr B_(2) O_(3) (s) , Delta H = 0 1273 k J//mol (ii) H_(2) (g) + ((1)/(2)) O_(2) (g) rarr H_(2) O (l), Delta H = -286 kJ//mol (iii) H_(2) O (l) rarr H_(2)O (g) Delta H = 44 kJ//mol (iv) 2B (s) + 3H_(2) (G) rarr B_(2) H_(6) (g), Delta H = 36 kJ//mol
View SolutionFrom the following data, mark the option (s) where DeltaH is correctly written for the given reaction. Given: H^(+)(aq)+OH^(-) (aq) rarr H_(2)O(l)" "DeltaH= -57.3 kJ DeltaH_("solution") of HA(g)= -70.7 kJ/mol Delta_("solution") of BOH(g)=20 kJ/mol DeltaH_("ionization") of HA=15 kJ/mol & BOH is a strong base
View SolutionFor the reaction : C2H5OH(l)+3O2(g)→2CO2(g)+3H2O(g)
if ΔU∘=−1373kJmol−1 at 298K. Calculate ΔH∘
View SolutionCalculate the heat of formation of KCl from the following data : {:((i),KOH(aq)+HCl(aq)rarr KCl(aq)+H_(2)O(l)",",,Delta H=-57.3 kJ mol^(-1)),((ii),H_(2)(g)+(1)/(2)O_(2)(g)rarr H_(2)O(l)",",,Delta H=-286.2 kJ mol^(-1)),((iii),(1)/(2)H_(2)(g)+(1)/(2)Cl_(2)(g)+aq rarr HCl(aq)",",,Delta H=-164.4 kJ mol^(-1)),((iv),K(s)+(1)/(2)O_(2)(g)+(1)/(2)H_(2)(g)+aq rarr KOH(aq)",",,Delta H=-487.4 kJ mol^(-1)),((v),KCl(s)+aq rarr KCl(aq)",",,Delta H=+18.4 kJ mol^(-1)):}
View SolutionCalculate the heat of formation of KCl from the following data : (i) KOH (aq) +HCl(aq) rarr KCl(aq) + H_(2) O(l) , Delta H = - 57.3 kJ mol^(-1) (ii) H_(2)(g) +(1)/(2) O_(2)(g) rarr H_(2)O(l), Delta H = - 286.2kJ mol^(-1) (iii) (1)/(2) H_(2)(g) +(1)/(2)Cl_(2)(g) +aq rarr HCl(aq), Delta H = - 164.4 kJ mol^(-1) (iv) K(s) + (1)/(2) O_(2) (g) +(1)/(2) H_(2)(g) +aq rarr KOH (aq), Delta H = - 487 . 4 kJ mol^(-1) (v) KCl(s) + aq rarr KCl (aq), Delta H = + 18.4 kJ mol ^(-1)
View SolutionCalculate Delta H ^(298) for the reaction : 2Li (s) + 2H_(2)O(l) rarr 2Li^(+) ( aq) + 2OH^(-) (aq) + H_(2)(g) Given that the standard enthalpies of formation of Li^(+)(aq), OH^(-)(aq) and H_(2)O(l) are - 278.5, - 228.9 and - 285.8 kJ mol^(-) respectively ( all at 298 K )
View SolutionGiven that (at 25^(@)C) : (1)/(2)H_(2)(g)+(1)/(2)O_(2)(g) to OH(g), Delta H^(0)= 38.95 " kJ mol"^(-1) H_(2)(g)+(1)/(2)O_(2)(g) to H_(2)O (g), Delta H^(0) = -241.8 " kJ mol"^(-1) H_(2)(g) to 2H(g), Delta H^(0)= 436.0 " kJ mol"^(-1) O_(2)(g) to 2O (g), Delta H^(0)= 498.3 " kJ mol"^(-1) Calculate Delta H^(0) for the following reaction - (a) OH(g) to H(g)+O(g) (b) H_(2)O(g) to 2H(g)+O(g)
View SolutionCalculate the bond strength of O-H bond in O−H bond in H2O(g) from the following data :
H2(g)→2H(g),ΔH0=436kJ⋅mol−1
12O2(g)→O(g),ΔH0=249kJ⋅mol−1
H2(g)+12O2(g)→H2O(g),ΔH0=−241kJ⋅mol−1
View SolutionGiven C (graphite) + O_(2) (g) to CO_(2) (g) Delta ""_(r) H^(0) = -393.5 k J * mol^(-1) H_(2) (g) + (1)/(2) O_(2) (g) to H_(2) O (l) , Delta ""_(r) H^(0) = -285.8 kJ * mol^(-1) CO_(2) (g) + 2 H_(2) O(l) to CH_(4) + 2 O_(2) (g) , Delta ""_(r)H^(0) = + 890 . 3 kJ * mol^(-1) Based on the above thermochemical equations , the value of Delta ""_(r) H^(0) at 298 K for the reation C(graphite) + 2 H_(2) (g) to CH_(4) (g) will be -
View SolutionEnthalpy of formation of NH3 is -X kJ and ΔHH−H, ΔHN−H are respectively Y kJmol−1 and Z kjmol−1. The value of ΔHN=Nis
View Solution
