Statement-1: Me-C= and Me−→N=C are not functional isomers.
Statement-2: As in HCN cyanide and isocyanide groups are interconvetible into each other and remains in equilibium.
Statement-1 is True, Statement-2 is True, Statement-2 is a correct explanation for Statement-1.
Statement-1 is True, Statement-2 is True, Statement-2 is NOT a correct explanation for Statement-1.
Statement-1 is True, Statement-2 is False.
Statement-1 is False, Statement-2 is True.
The correct Answer is:d
Me−C≡NandM−N−−→=C are functional isomers but H−C≡NandH−N=C are tautomers.
Topper's Solved these Questions
- ISOMERISMBOOK - GRB PUBLICATIONCHAPTER - ISOMERISMEXERCISE - MULTIPLE OBJECTIVE TYPE
71 Videos
- ISOMERISMBOOK - GRB PUBLICATIONCHAPTER - ISOMERISMEXERCISE - COMPREHENSION TYPE
25 Videos
- ISOMERISMBOOK - GRB PUBLICATIONCHAPTER - ISOMERISMEXERCISE - SUBJECTIVE TYPE
67 Videos
- GASEOUS STATEBOOK - GRB PUBLICATIONCHAPTER - GASEOUS STATEEXERCISE - Exercise
530 Videos
- MOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMSBOOK - GRB PUBLICATIONCHAPTER - MOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMSEXERCISE - Subjective type
145 Videos
Similar Questions
Knowledge Check
Statement I: Me−C≡N and Me−N→=C are not functional isomers.
Statement II: As in HCN cynide and isocyanide groups are iterconvertible into each other and remains equilibrium .AIf both statement-I and statement II are true & the statement II is a correct explanation of the statement IBIf both statemetn -I and Statement II are True and Statement II is not a correct explanation of the Statement ICIf Statement -I is True but the Statement -II is falseDIf Statement -I is false but the Statement -II is True.Statement-1: Cyanides are more polar than isocyanides.
and
Statement-2: Isocynides show addition of oxygen or sulphur with HgO or HgS respectively.AStatement-1 is true, Statement-2 is true, statement-2 is a correct explanation for statement-9BStatement-1 is true, statement-2 is true, statement-2 is not a correct explanation for statement-9CStatement-1 is true, statement-2 is FalseDStatement-1 is False, Statement-2 is True.C2H5−OH and _________ are the functional group isomers .
AC2H5−O−CH3BCH3−O−CH3CC2H5−O−C2H5DCH3−OH
Statement-1: Me−⊕CH2 is more stable than MeO −CH⊕2
Statement-2: Me is a+I group where as MeO is a –I group.
View SolutionStatement -1: Methyl isocyanide has higher boiling point than methyl cyanide. And
Statement -2: Methyl cyanide has co-ordinate bond between N and C.
View SolutionStatement-1: Unsymmetrical ketone reacts with HCN to give (dil) mixture of carbohydrine.
Statement-2: Cyanide ion is amident nucleophile.
View SolutionStatement-1: All C-C bonds are equal in [10]-Annulene.
and
Statement-2: [10]-Annulene is a non aromatic compound
View SolutionStatement-1:
 Expected major product from the above reaction
Expected major product from the above reaction 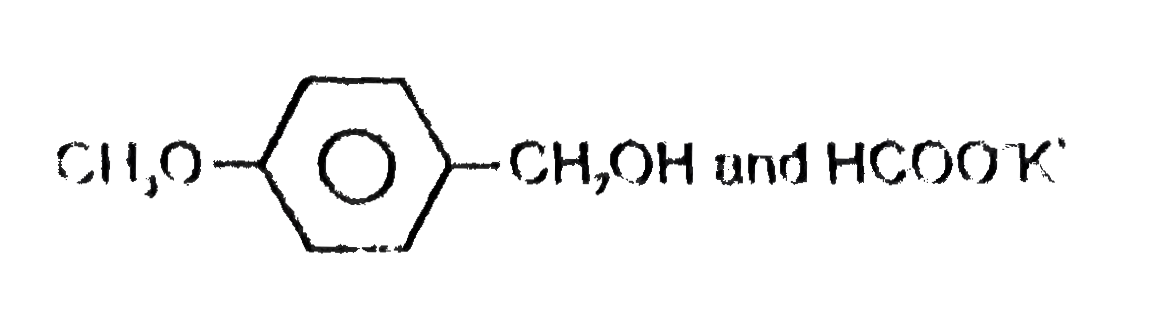
Statement-2:With increase in electrophilicity on gt C=O group, rate of attack of nuecleophile increases.
View Solution