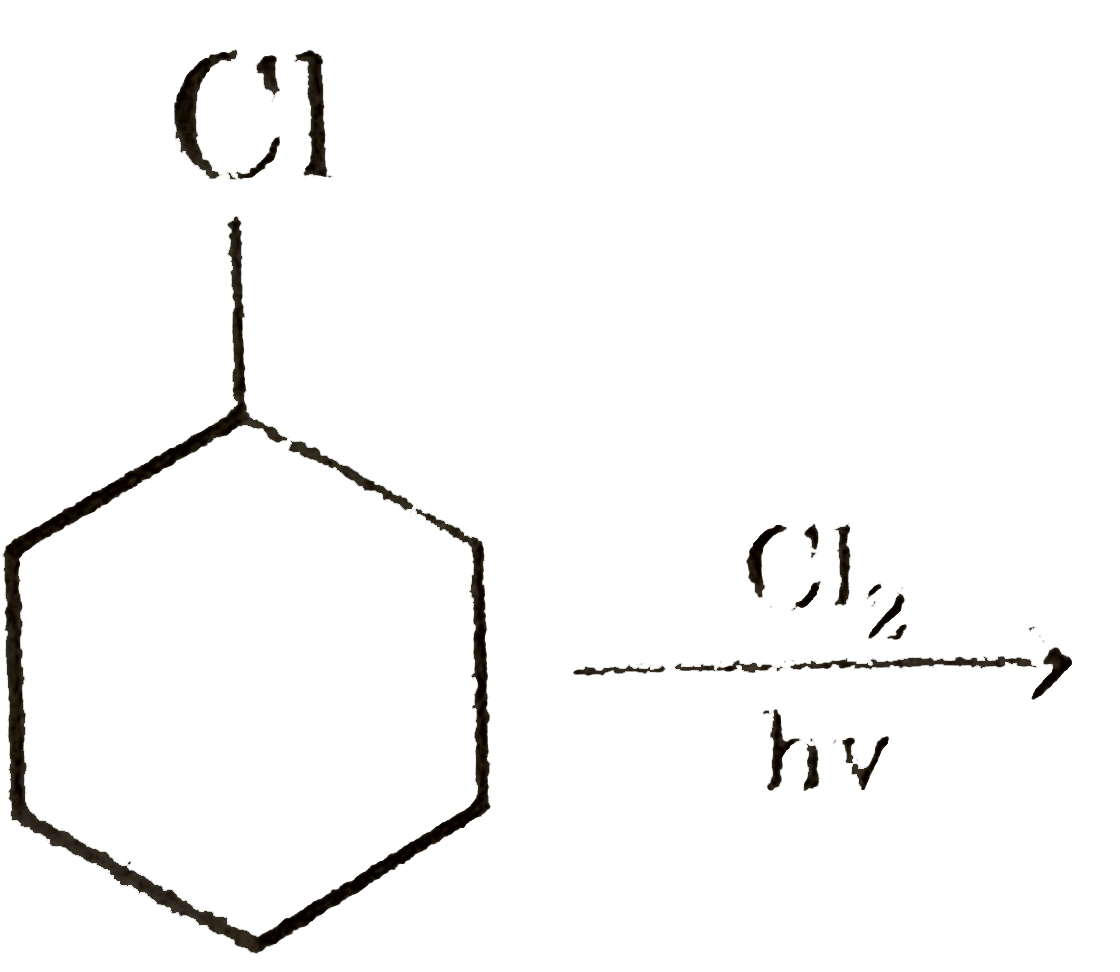
Cl2−−→hv number of theretically possible dichloro products are:
The correct Answer is:9
The following Reaction are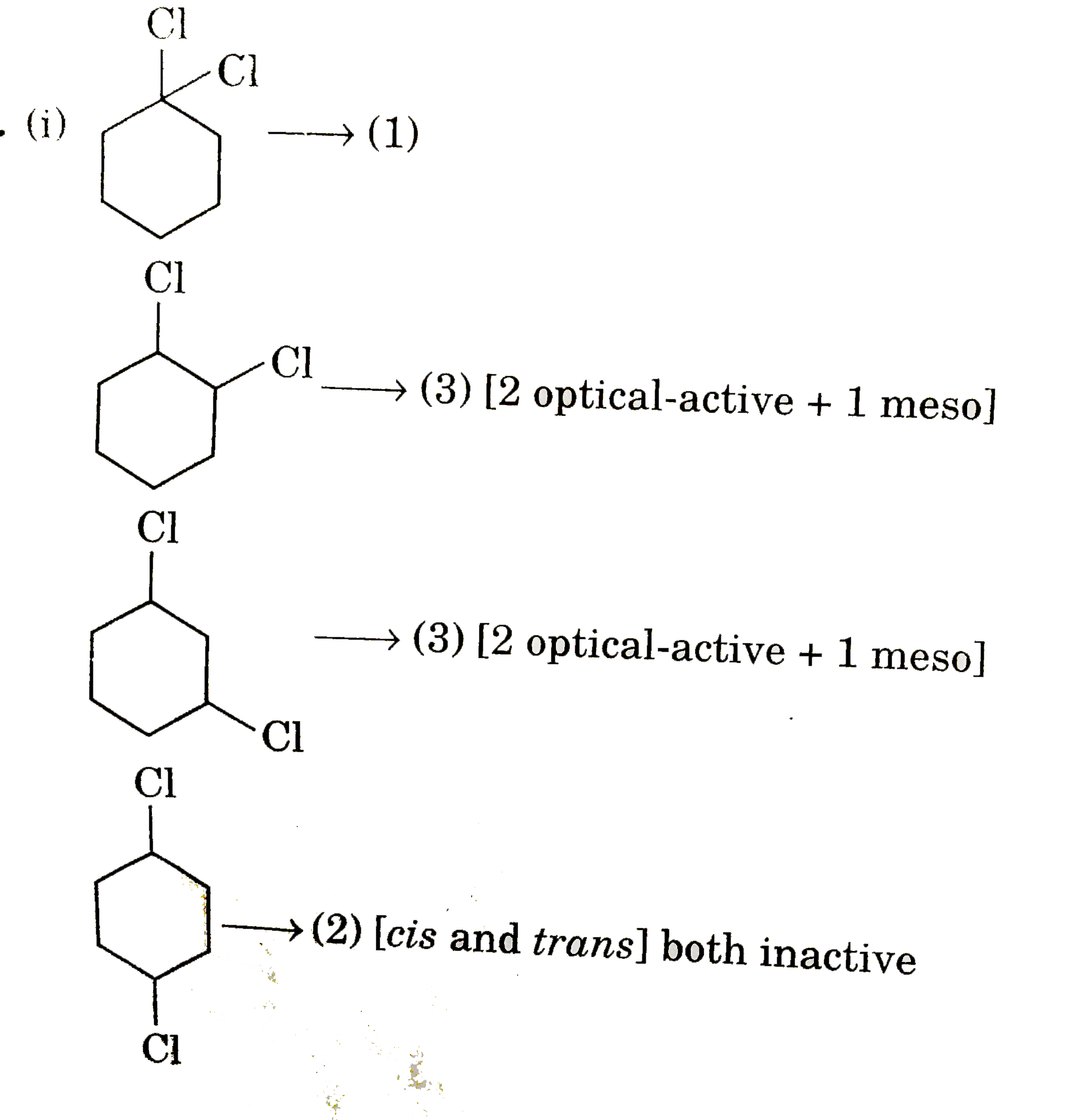
Topper's Solved these Questions
- ISOMERISMBOOK - GRB PUBLICATIONCHAPTER - ISOMERISMEXERCISE - MATCH THE COLUMN TYPE
22 Videos
- GASEOUS STATEBOOK - GRB PUBLICATIONCHAPTER - GASEOUS STATEEXERCISE - Exercise
530 Videos
- MOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMSBOOK - GRB PUBLICATIONCHAPTER - MOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMSEXERCISE - Subjective type
145 Videos
Similar Questions
Knowledge Check
n-Pentane Cl2−−→hv(A) (no. of total product)
A2B3C4D5Consider the following reaction
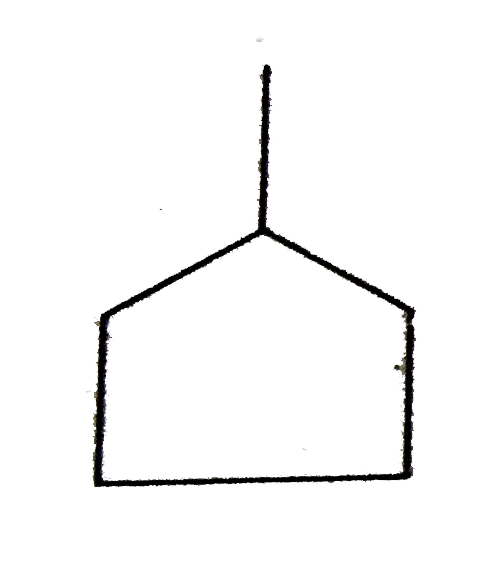 Cl2−−→hv total number of mono chlorinated product =x
Cl2−−→hv total number of mono chlorinated product =x
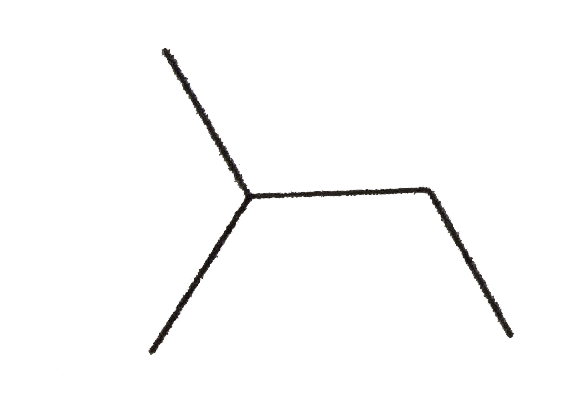 Cl2−−→hvtotal number of mono chlorinated(Exluding stereoisomer)product=y
Cl2−−→hvtotal number of mono chlorinated(Exluding stereoisomer)product=y
identify value of x+y?A13B14C12D16 Cl2−−→hv A mixture of all isomers possible from the mono-chlorination the structures is subjected to fractional distillation, then how many fractions will be obtaine A2B3C4D5
Cl2−−→hv A mixture of all isomers possible from the mono-chlorination the structures is subjected to fractional distillation, then how many fractions will be obtaine A2B3C4D5
Reaction-1 : CH3−CH2−CH2−CH2−CH3Cl2−−−−−→hv Optically active monochloro products (s) are (R) .
Reaction-2 :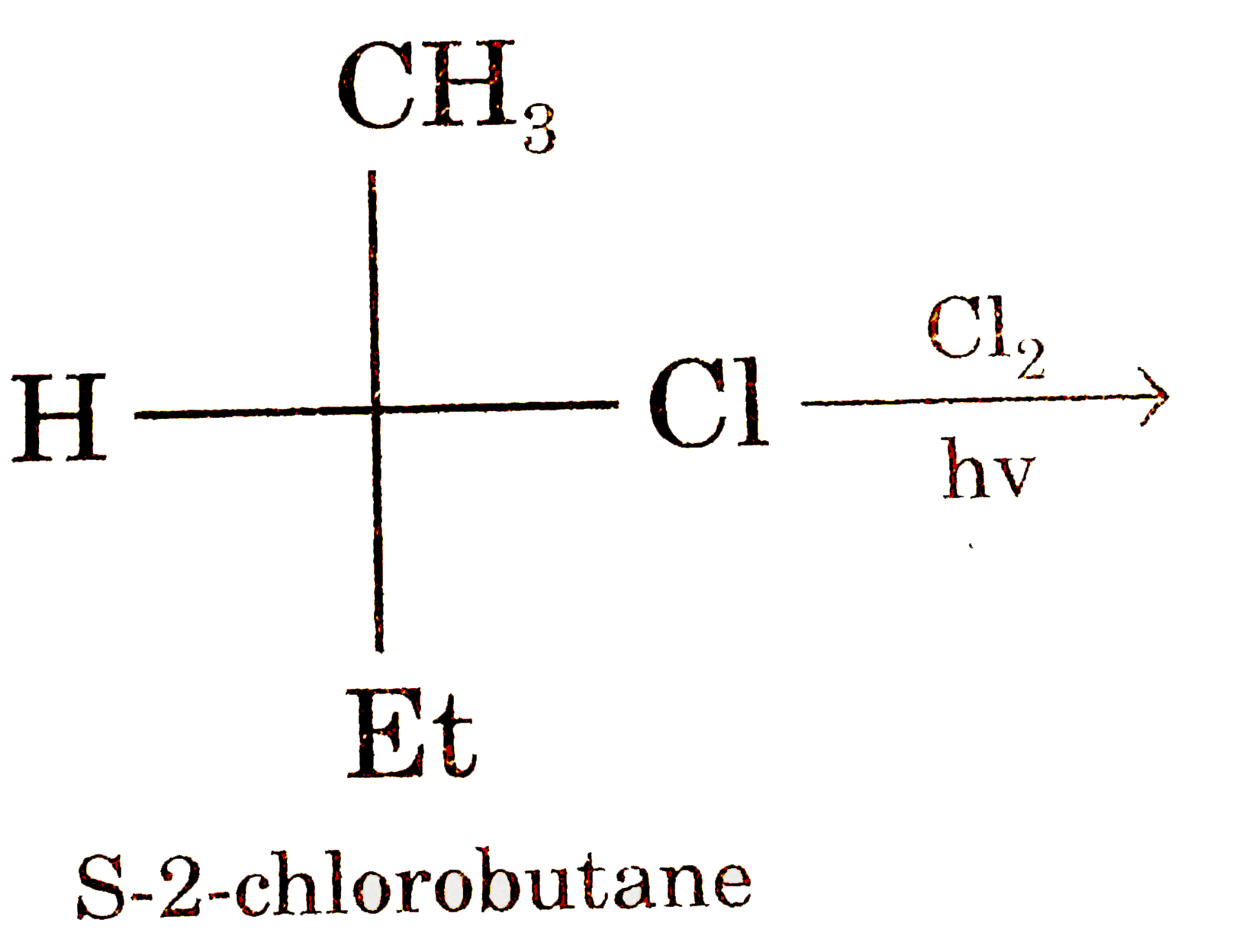 Optically active di-chloro products (s) are (S).
Optically active di-chloro products (s) are (S).
Reaction-3 :
Optically active di-chloro product (s) are (P).
Reaction-4 : 2-methoxy propaneCl2−−−−−→hv Optically active monochloro product (s) are (Q).
What is the sum of P+Q+R+S?
View Solution Br2/hv−−−−→Product
Br2/hv−−−−→Product
View SolutionCH3−CH∣CH3−CH2−CH2−CH3Cl2−−→hv
Mono-chloro product (inculding steroisomers) are :
View SolutionCH3CH2CH2Cl2−−→hv(A)+(B)(Monochlorination products )
the appoximate ratio of percentage yields of ( A) and (B) formed in the above reaction is :
View SolutionCH3CH2CH2CH3Cl2−−→hvA+B(monochlorination products)
The approximate ratio of percentage yields of A and B formed in the above reaction is
View Solution

