Compound A (C7H8O) is insoluble in water , dilute HCL and aqueous NaHCO3 it dissolves is dilute NaOH. When A is treated with Br2 water it is converted into a compound C7H5Obr3 reapidly . The structure of A is :
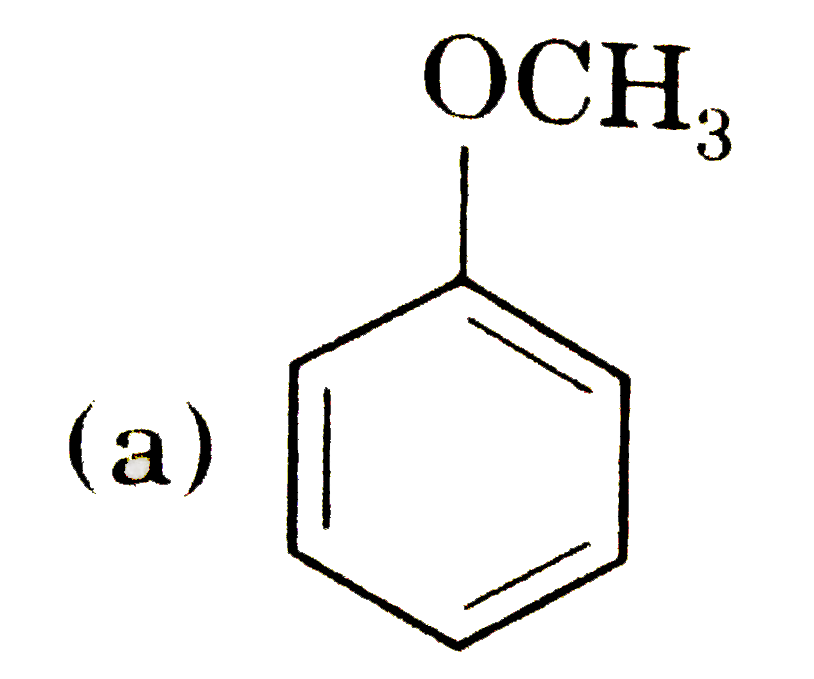

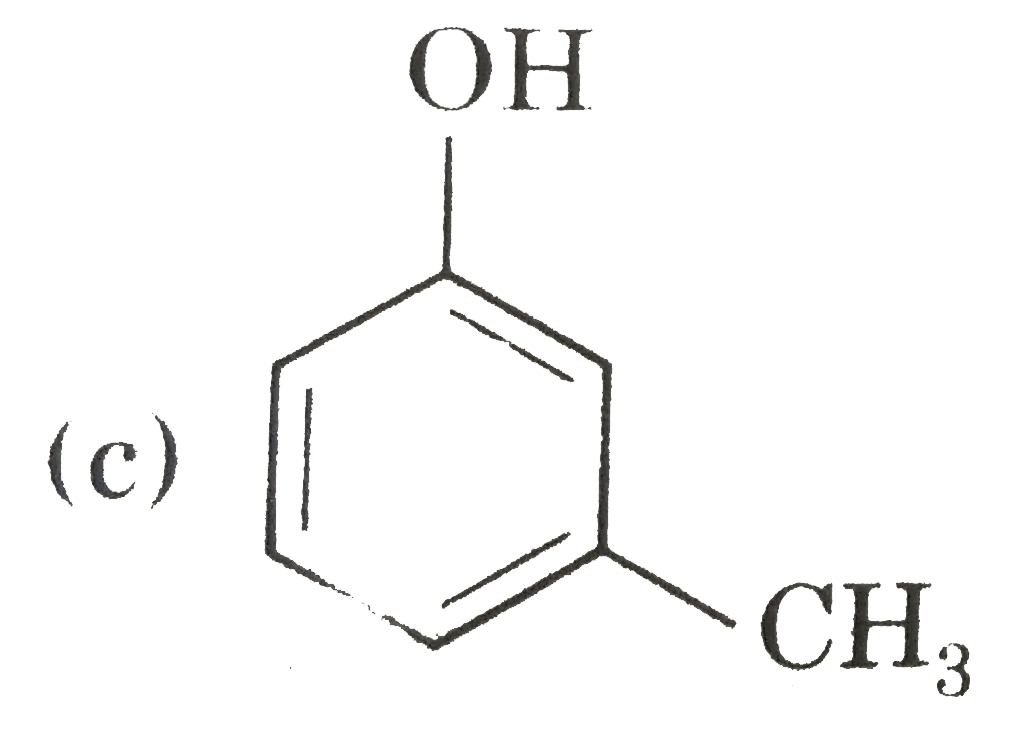
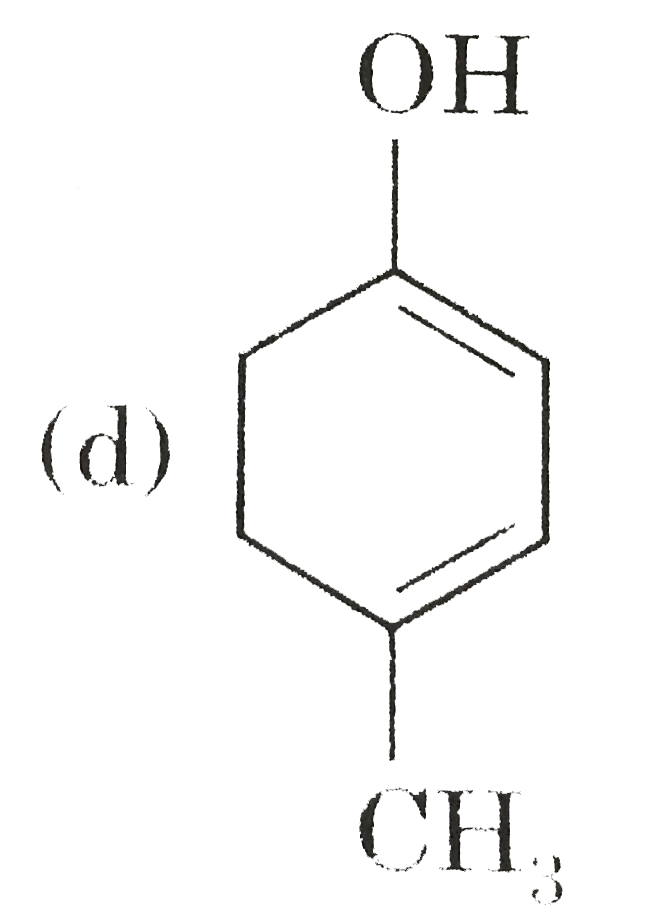
The correct Answer is:b
Topper's Solved these Questions
- BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEBook:GRB PUBLICATIONChapter:BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEExercise:REASONING TYPE
Explore 27 Videos
- BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEBook:GRB PUBLICATIONChapter:BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEExercise:MULTIPLE OBJECTIVE TYPE
Explore 72 Videos
- ALCOHOLS AND ETHERSBook:GRB PUBLICATIONChapter:ALCOHOLS AND ETHERSExercise:level 62
Explore 1 Video
- CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSBook:GRB PUBLICATIONChapter:CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSExercise:Subjective type
Explore 19 Videos
Similar Questions
A compound 'A' (C7H8O) is insoluble in water, dilute HCl and aqueous NaHCO3 but soluble in dilute NaOH . When A is treated with Br2−H2O, it is converted into a compound of formula C7H5Obr3. Compound A is :
View SolutionCompound Y,C7H8O is insoluble in water, dil HCl and aqueous NaHCO3 it dissolves in dilute NaOH.When Y is treated with bromine water it is converted rapidly into a compound of formula C7H6OBr3.Identify the structure of Y
View Solution
Knowledge Check
Compound A (C7H8O) is insoluble in water, dilute HCl & aqueous NaHCO3, but it dissolves in dilute NaOH. When A is treated with Br2 water it is converted into a compound C7H5Obr3 rapidly. The structure of A is:
A B
B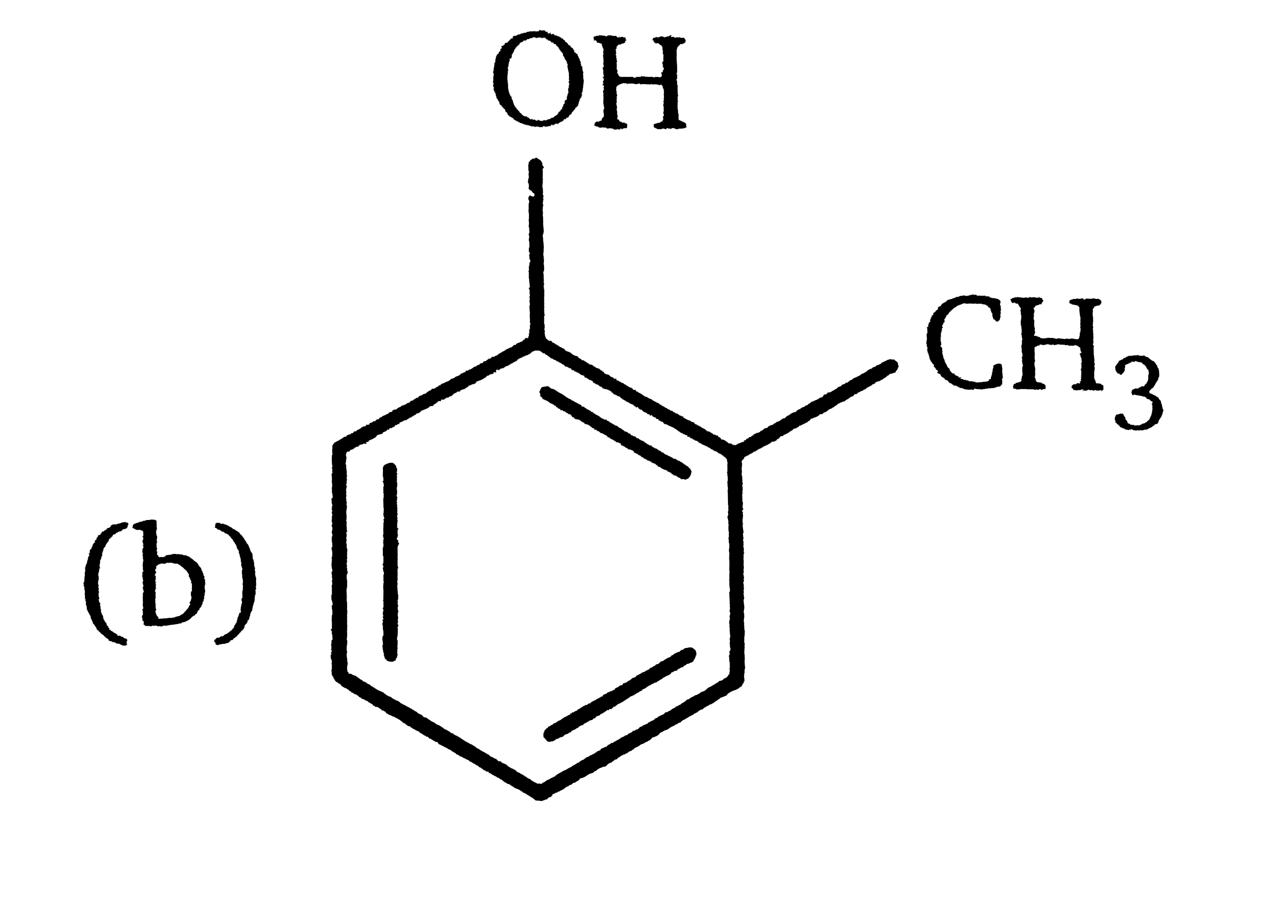 C
C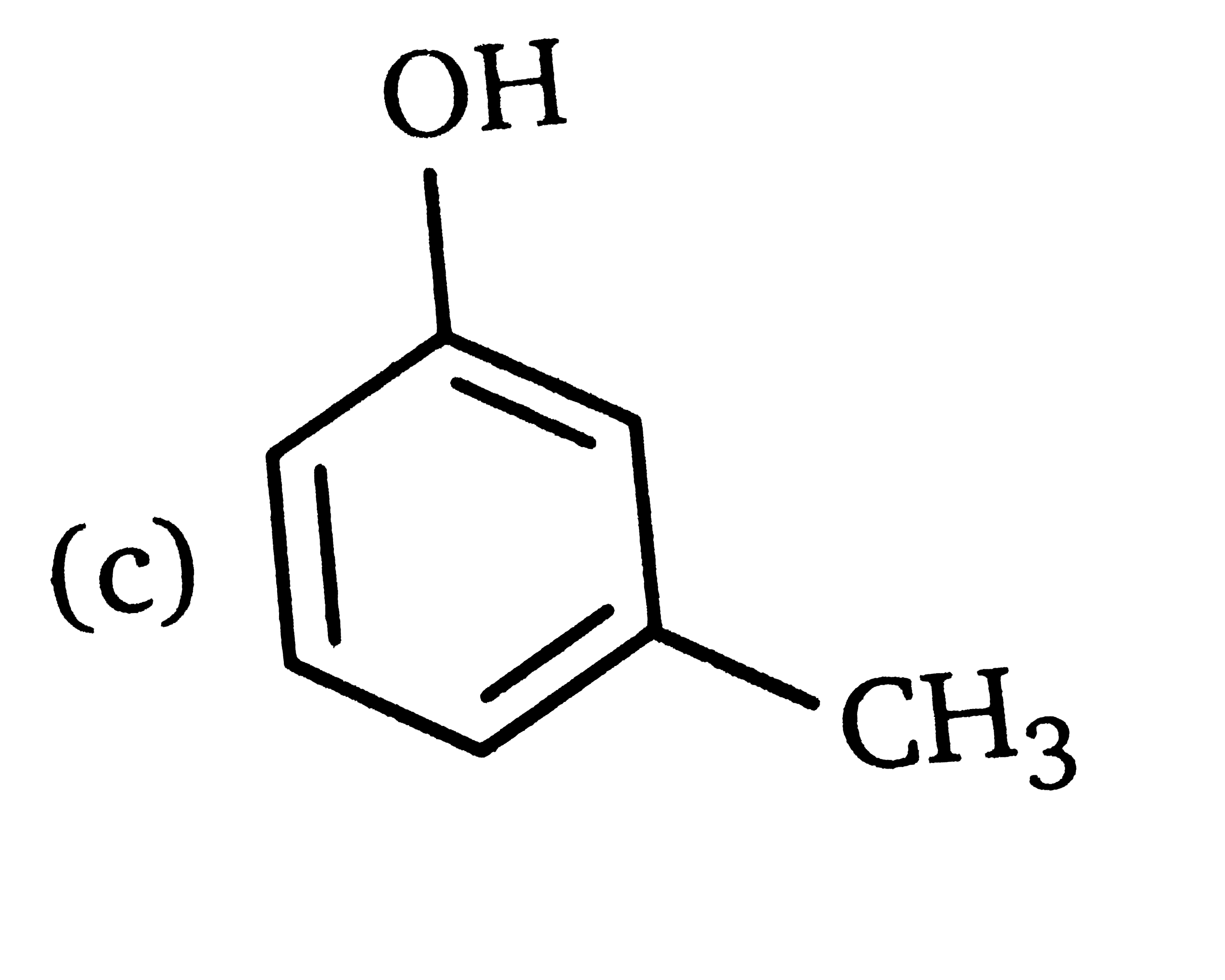 D
D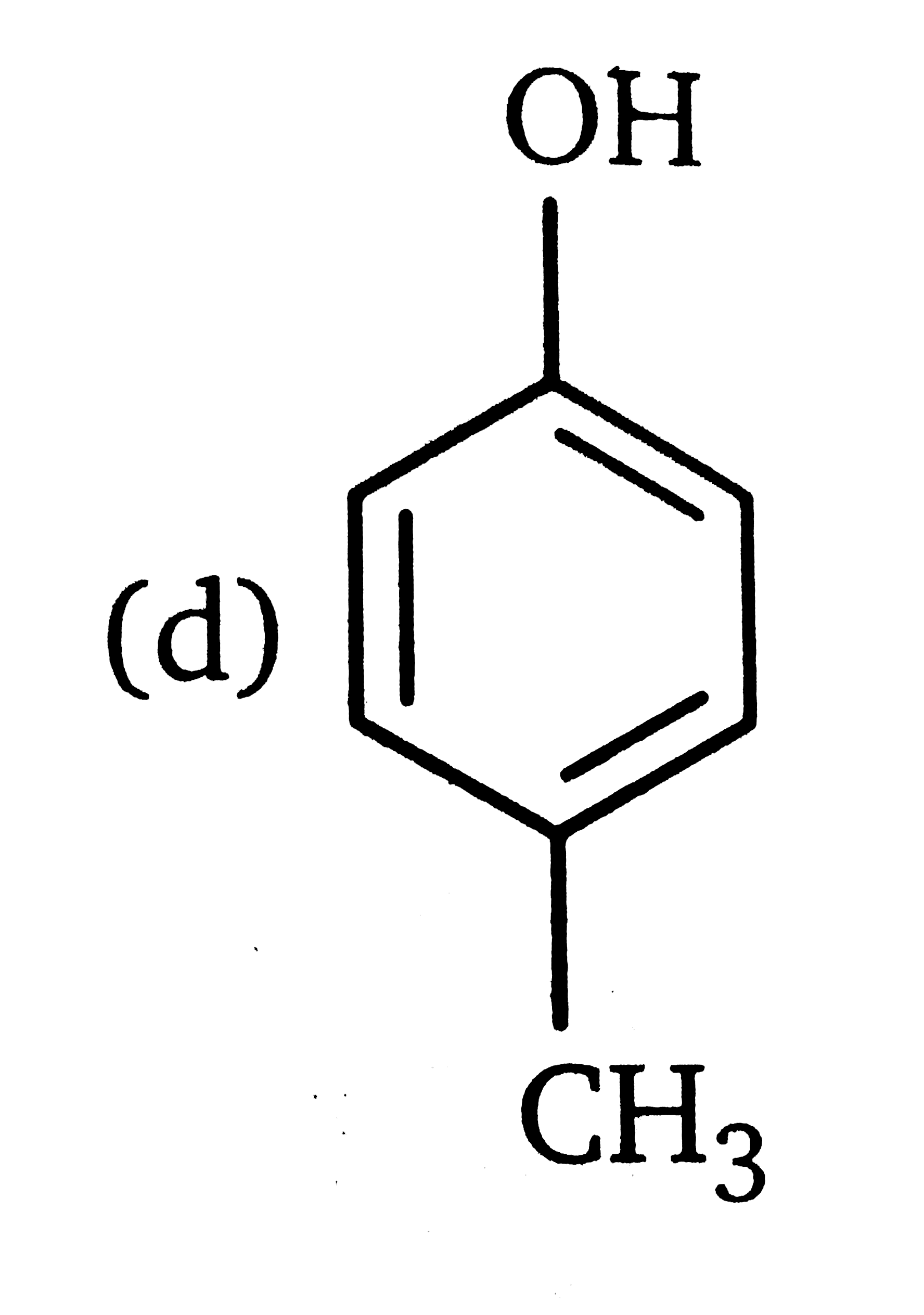
Compound A(C7H8O)is insoluble in water, dilute HCl & aqueous NaHCO3, it dissolves in dilute NaOH.When A is treatedwith Br2 water it is converted into a compound C7H5OBr3 rapidly. The structure ofA is
A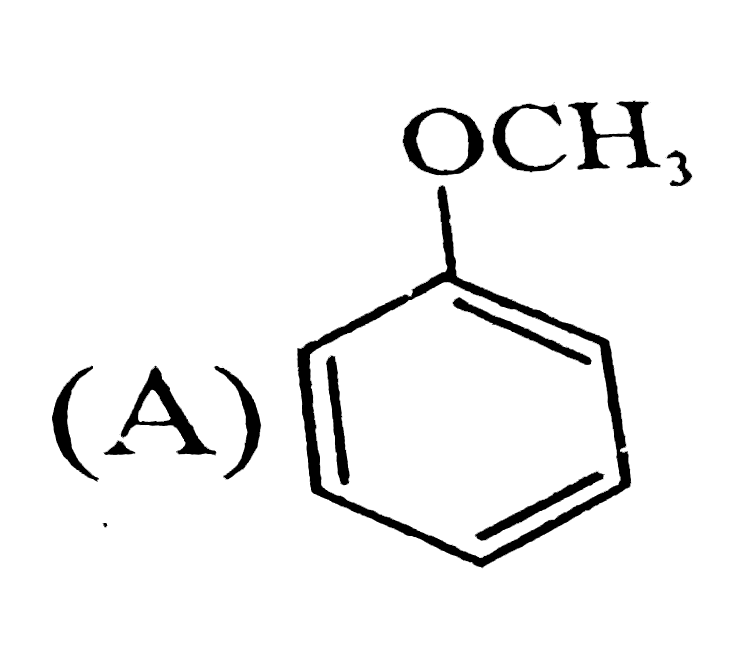 B
B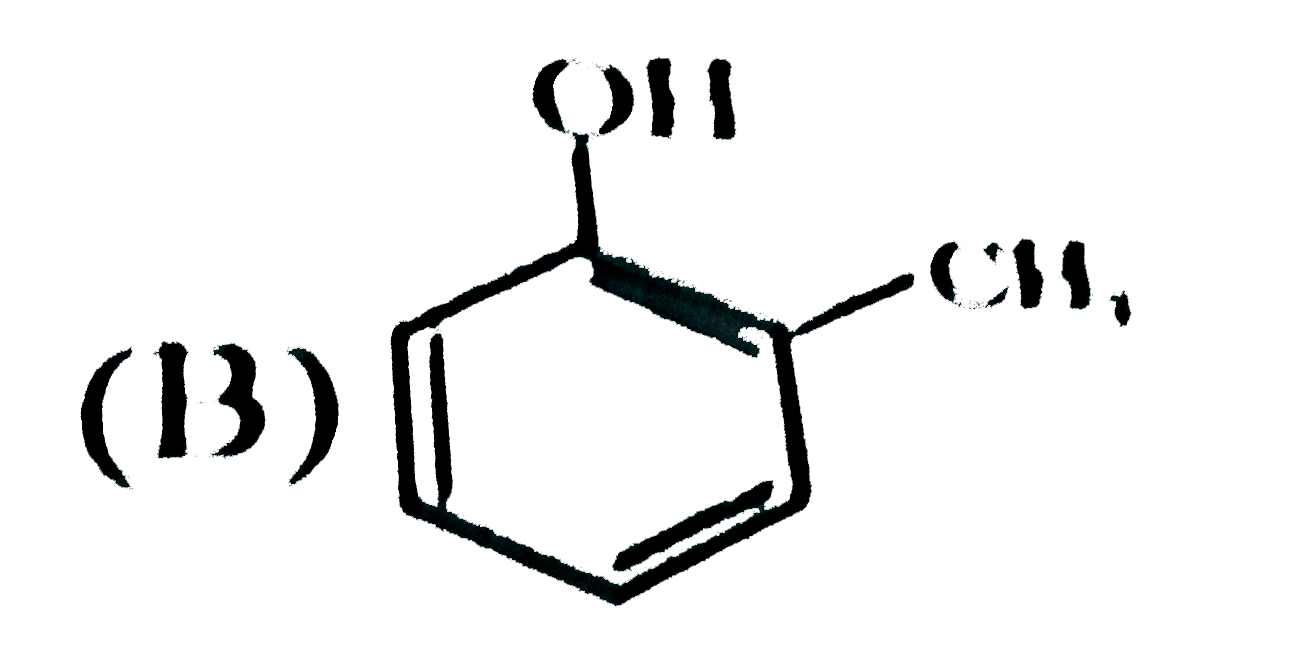 C
C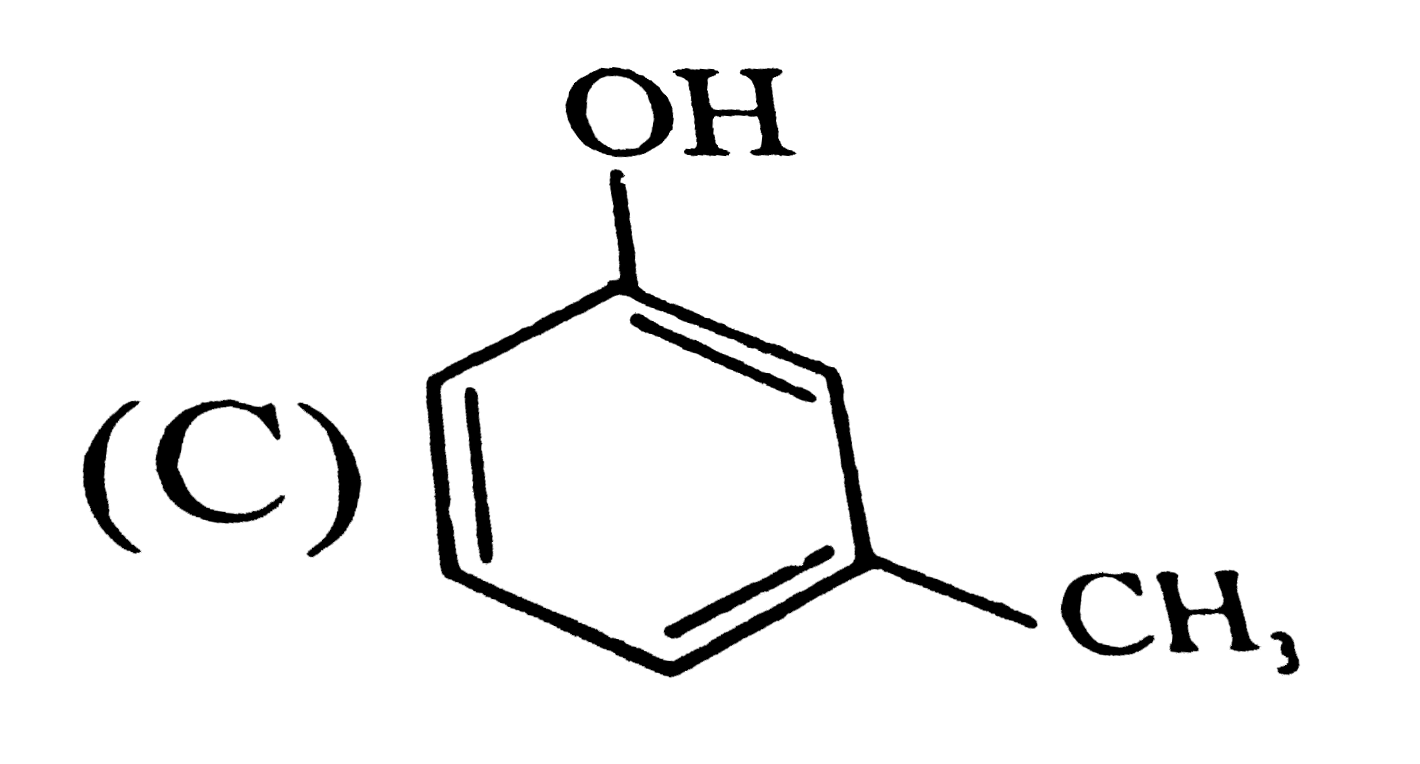 D
D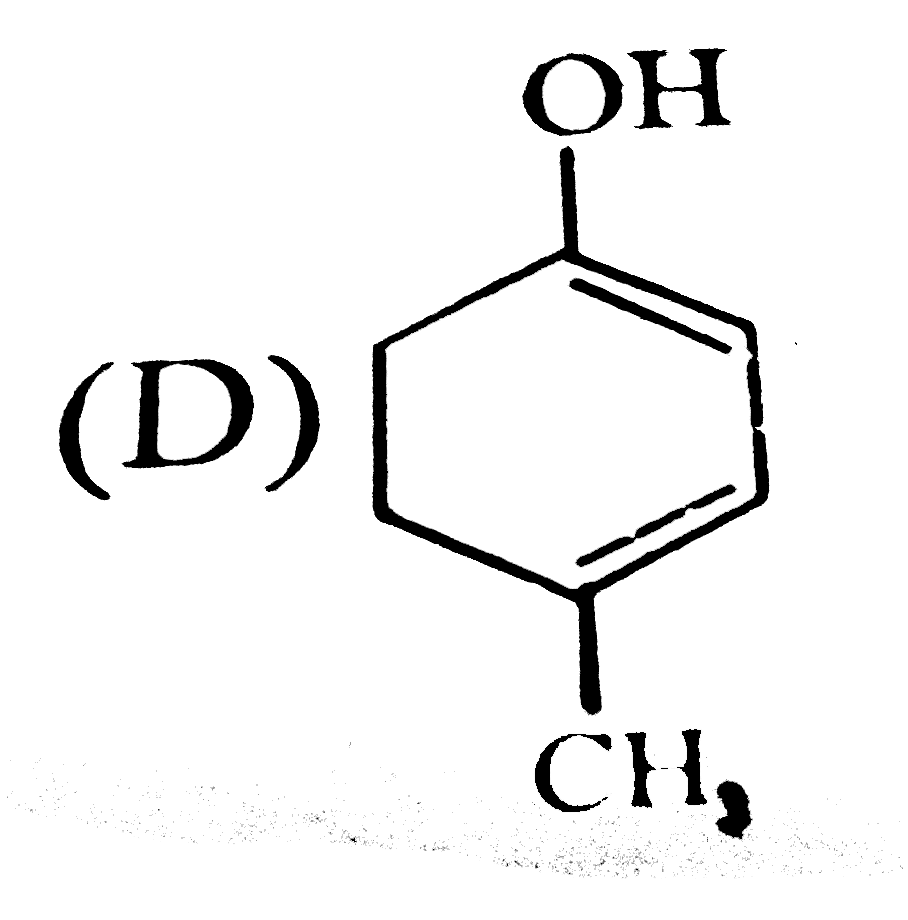
Compound A,C7H8O, is insoluble in water, dilute HCl, and aquenous NaHCO3, it dissolves in dilute NaOH. When A is treated with bromine water is is converted rapidly into a compound of formula C7H5Obr3. The structure of A is
A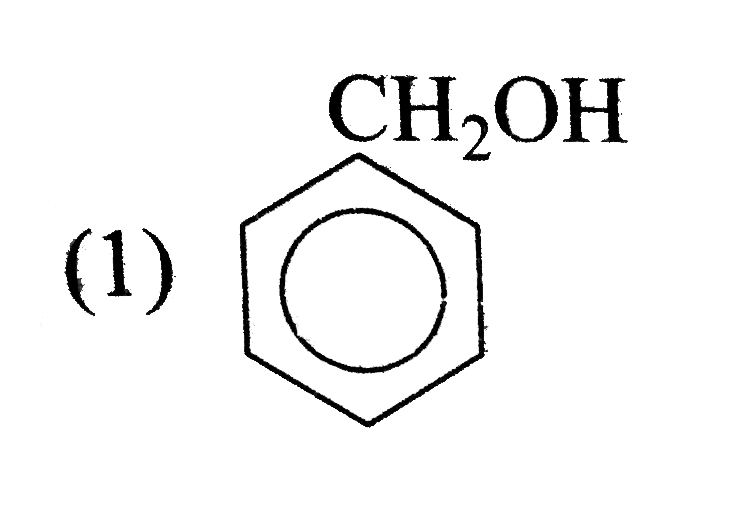 B
B C
C D
D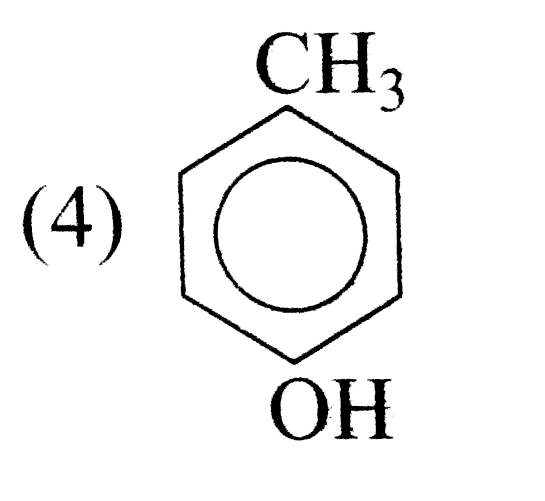
Compound 'P', C7H8O is insolution in water , dilute when HCI and NaHCO3 it disolves in dilite NaOH P is treated with Br2−H2O it converts rapidly into a compound of formula C7H5OBr3 Idenity structure of P?
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The structure of compound (B) would be :
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The structure of compound (B) would be :
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The most probable structure of compound A is :
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The most probable structure of compound A is :
View Solution

