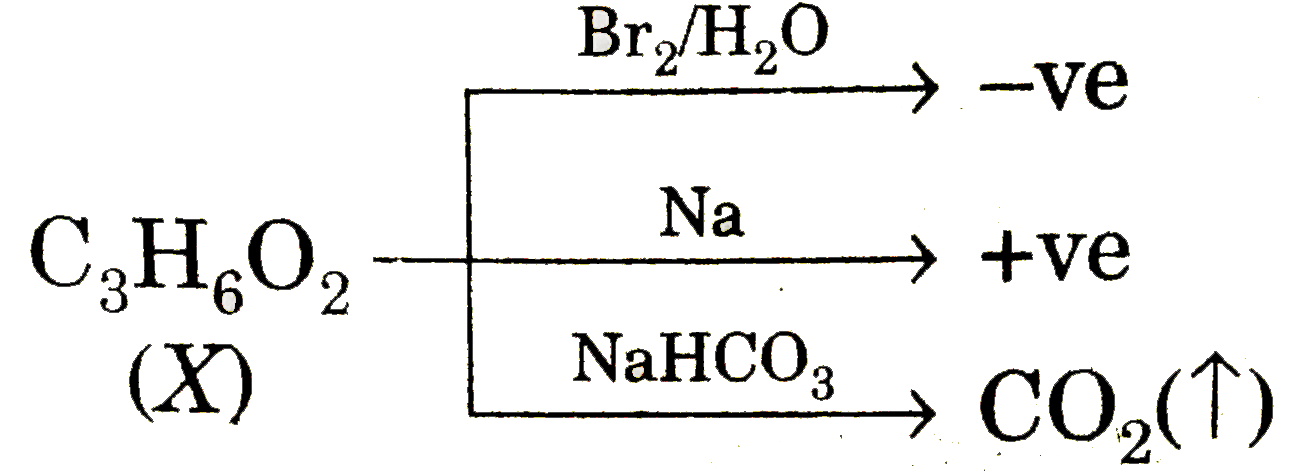
C3H6O2
CH3−CH2−COOH
CH3−CO−CH2−OH
The correct Answer is:a
Step by step video, text & image solution for C_(3)H_(6)O_(2) by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEBook:GRB PUBLICATIONChapter:BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEExercise:REASONING TYPE
Explore 27 Videos
- BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEBook:GRB PUBLICATIONChapter:BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFEExercise:MULTIPLE OBJECTIVE TYPE
Explore 72 Videos
- ALCOHOLS AND ETHERSBook:GRB PUBLICATIONChapter:ALCOHOLS AND ETHERSExercise:level 62
Explore 1 Video
- CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSBook:GRB PUBLICATIONChapter:CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSExercise:Subjective type
Explore 19 Videos
Similar Questions
Knowledge Check
A compound 'A' formula of C3H6CI2 on reaction with alkali can give 'B' of formula C3H6O or 'C' of formula C3H4.'B' on oxidatrtion gave a compound of the formula C3H6O2.'C' with dilute H2SO4 containing Hg2+ ion gave 'D' of formula C3H6O which with bromine and alkali gave the sodium salt of C2H4O2. Then 'A' is
ACH3CH2CHCI2BCH3CCI2CH3CCH2CICH2CH2CIDCH3CHCICH2CIThe inversion of cane sugar is represented by
C12H22O11+H2O→C6H12O6+C6H12O6
It is a reaction ofAsecond orderBunimolecularCpseudo unimolecularDnone of the threeThe oxidation state of Cr in [Cr(H2O)6]Cl3,[Cr(C6H6)2],andK2[Cr(CN)2(O)2(O2)(NH3)] respectively are :
A+3,0and+6B+3,0and+4C+3,+4and+6D+3,+2and+4
Select two homologous compounds from the following carbon compounds.
CH4OC2H6O2C2H6OC3H6O
View SolutionWhich two of the following compounds belong to same homologous series?
C2H6O2,C2H6O,C2H6,CH4O
View SolutionWith alkali C3H6CI2(A) gives CH3H6O(B) or C3H4(C).(C) reacts with dilute H2SO4 containing mercuric sulphate to giveC3H6O(D) which gives iodoform test.
View SolutionFor the reaction C12H22O11+H2OH+−−→C6H12O6+C6H12O6, write
(i) Rate of reaction expression (ii) rate law equation (iii) molecularity (iv) order of reaction
View SolutionThe oxidation states of
Cr in [Cr(H2O)6]Cl3.[Cr(C6H6)2] and
K2[Cr(CN)2(O)2(O2)(NH3)] respectively are
View Solution
