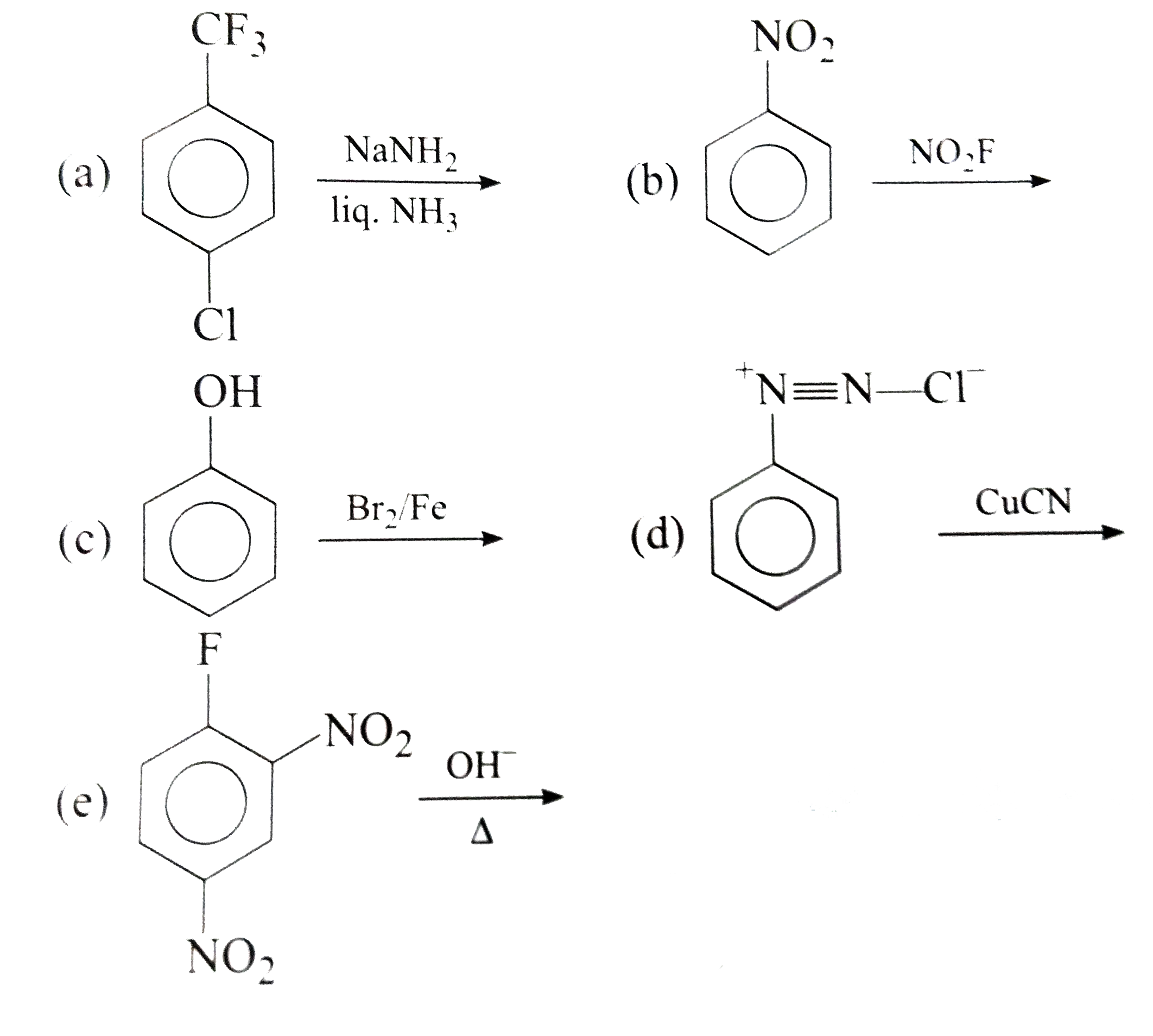
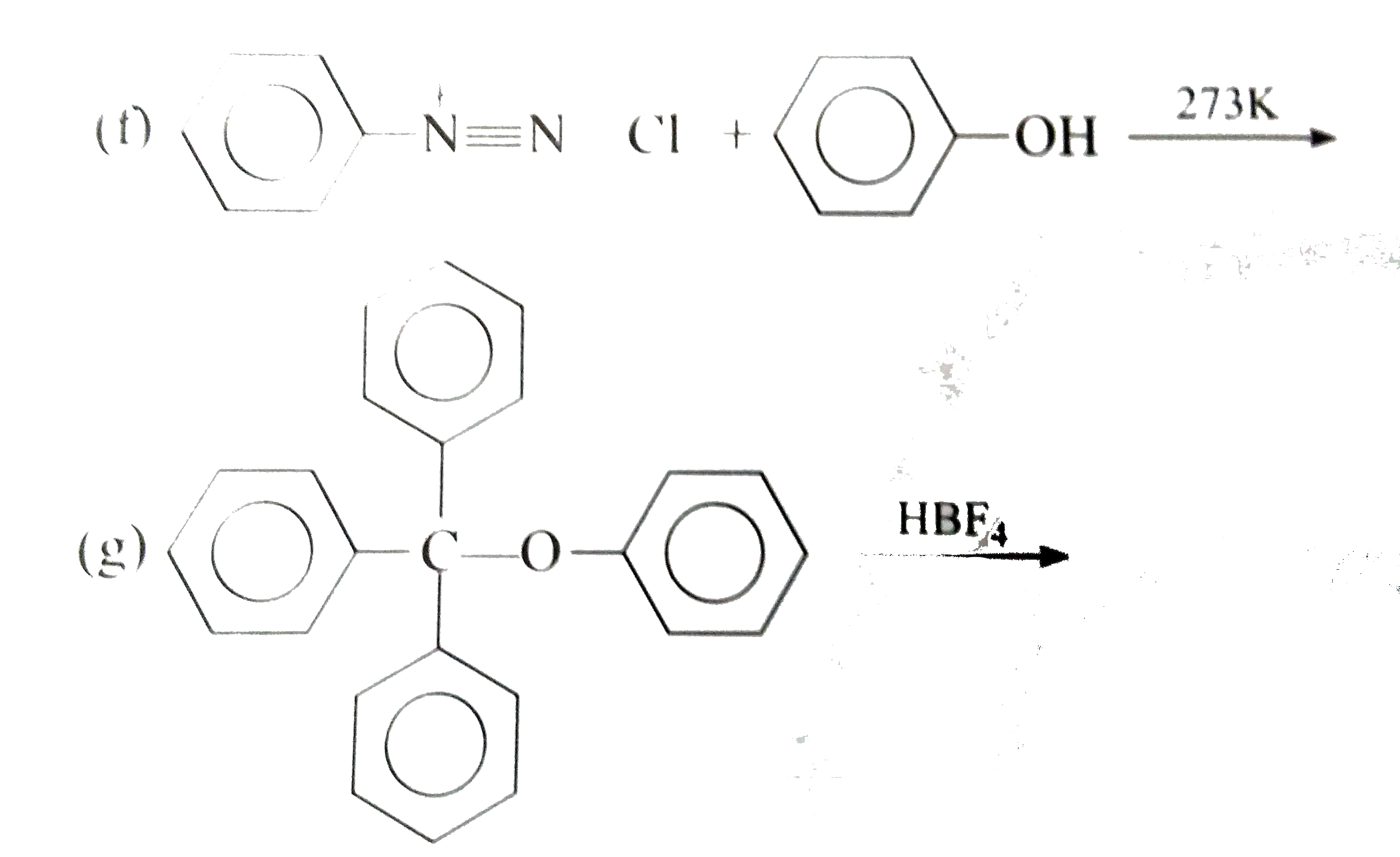
Find out number of reaction which involve electron deficient nitrogen during reaction mechanism.
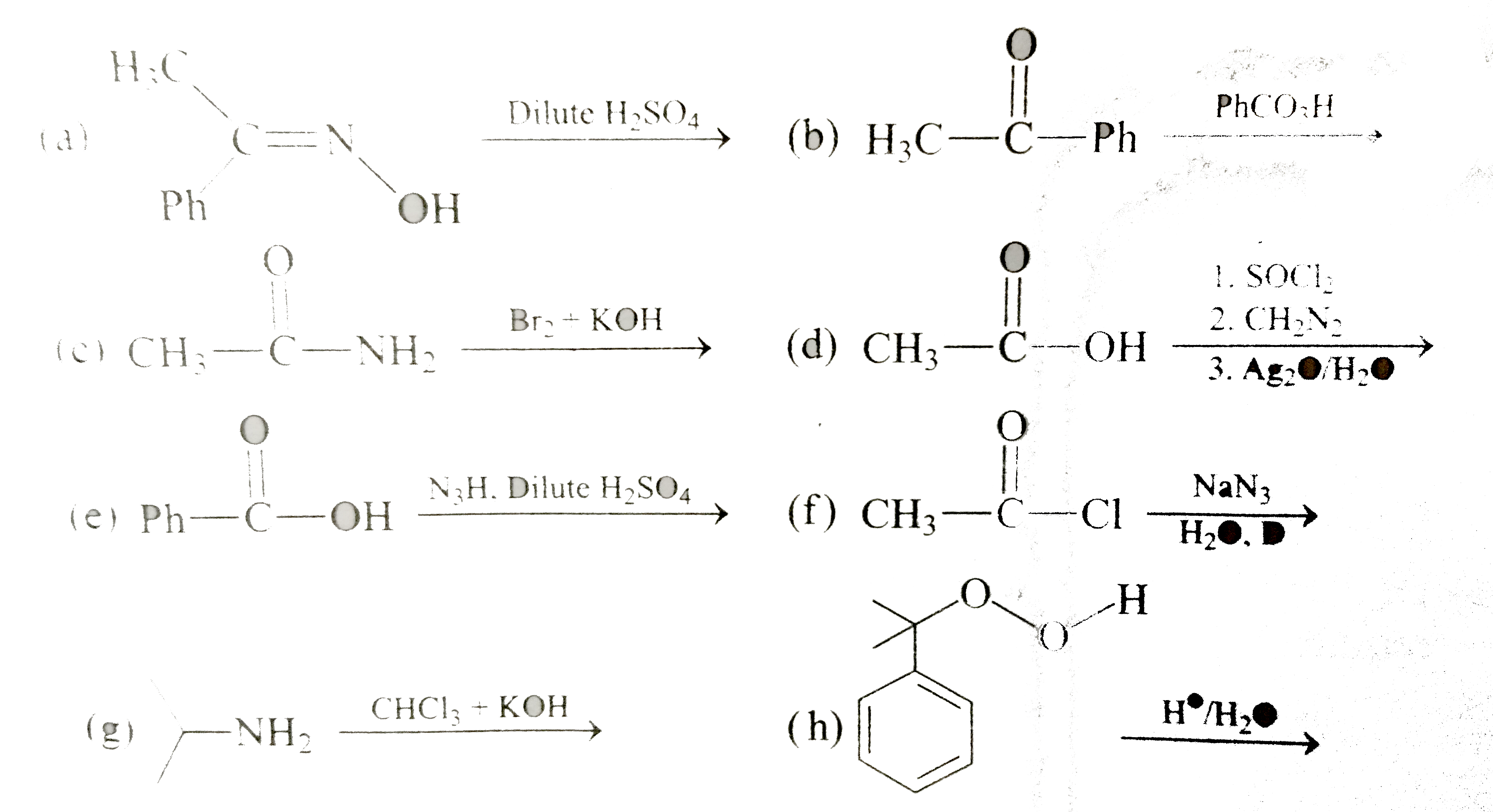
The correct Answer is:4
Step by step video, text & image solution for Find out number of reaction which involve electron deficient nitrogen during reaction mechanism. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Which of the following events does not occur during SN2 reaction mechanism?
ABack side attack of nucleophileBFormation of carbonium ionCOne step continuous processD100% inversion of configurationWhich type of reaction (s) is/are involved in the Cannizaro reaction ?
AReductionBOxidationCBoth (a) and (b)DNone of these
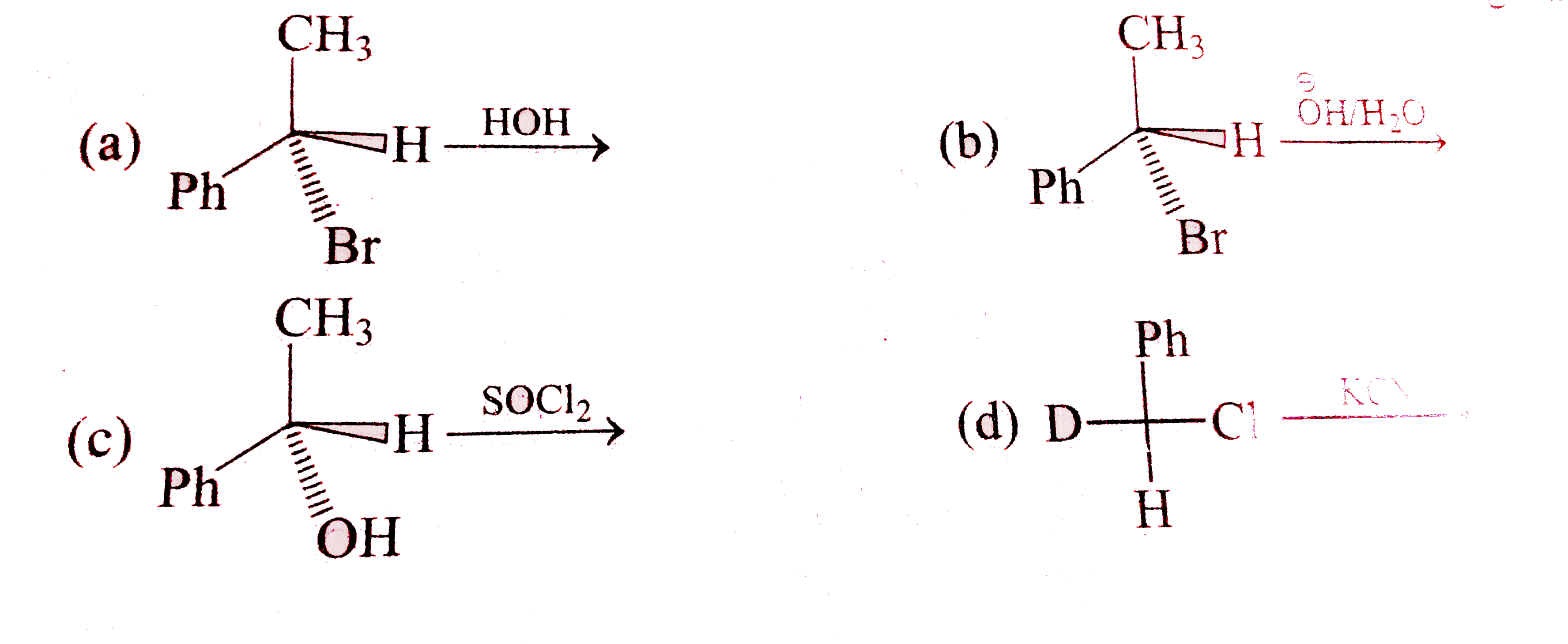
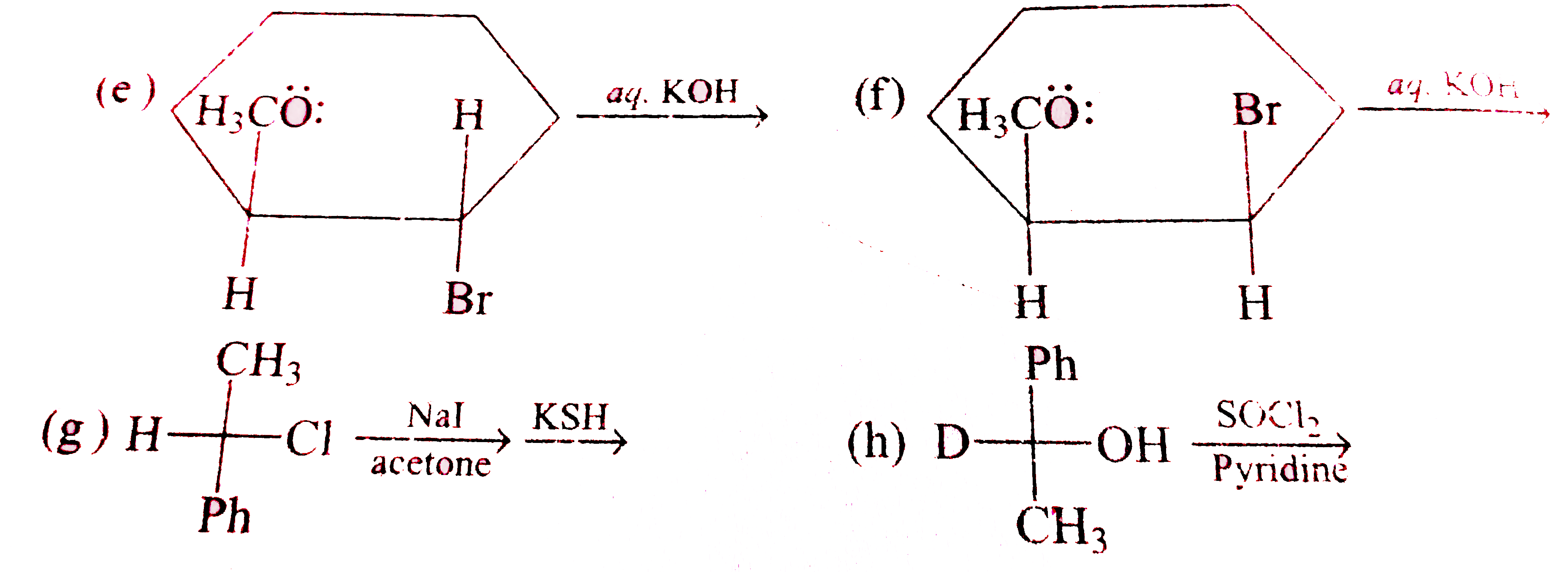
Find out number of reactions those proceed with retention of configuration.
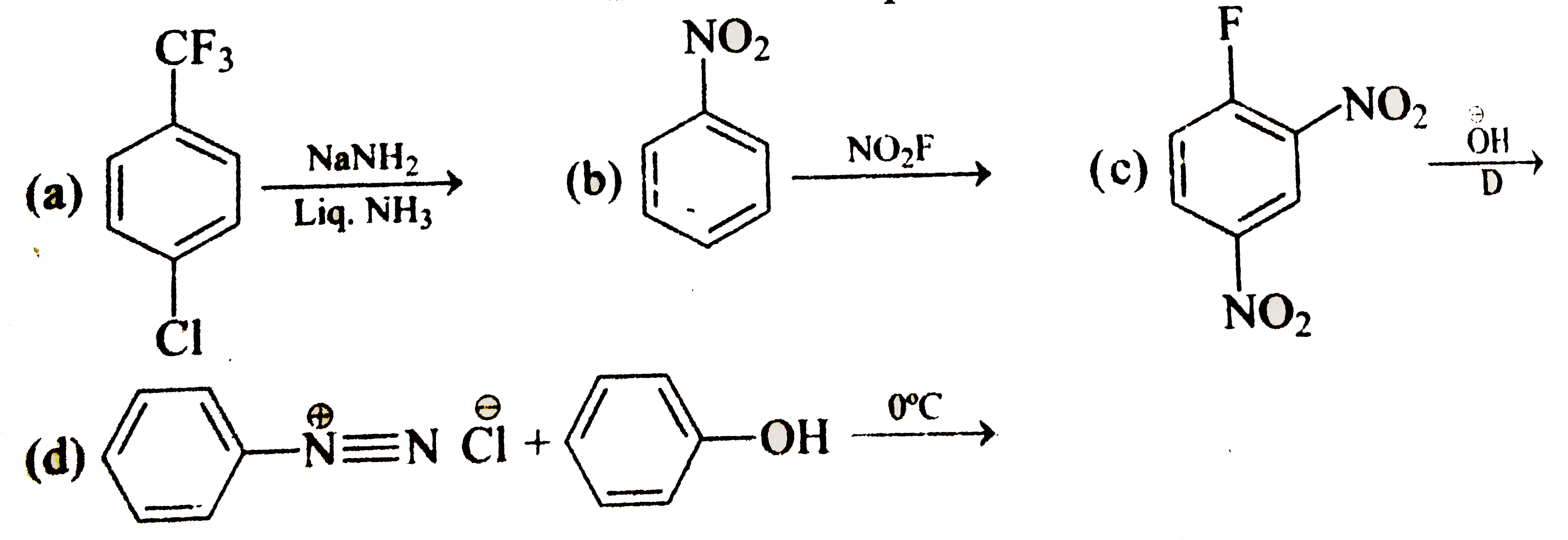
View SolutionFind out number of reaction that are electrophilic aromatic substitution in nature .
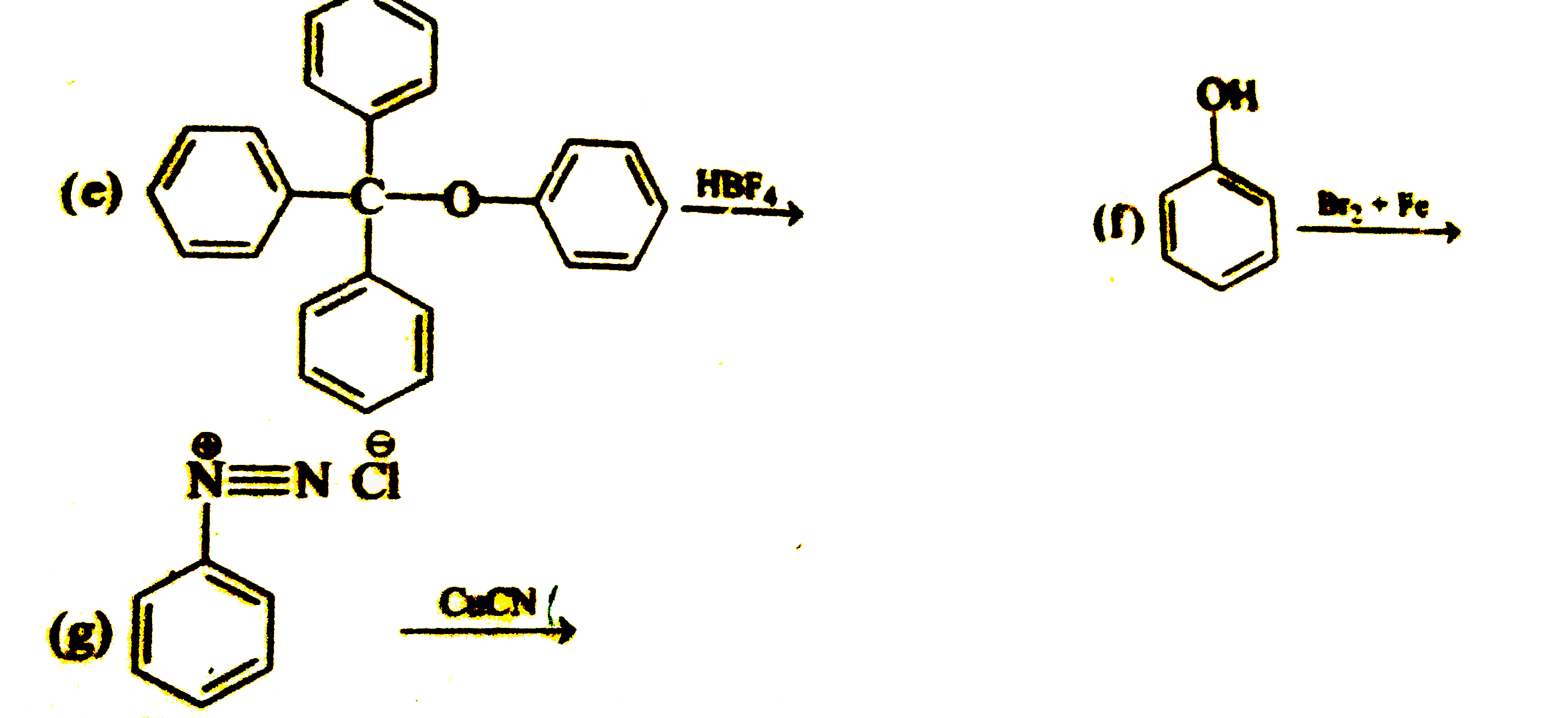
View SolutionBalancing redox reaction by ion-electron method
Chemical reactions which involve oxidation and reduction (redox reactions) can also be balanced by using the ion-electron method or half-equation method. This method of balancing redox reactions is based upon the principle that electrons lost during oxidation halfreaction of any redox reaction are equal to the electrons gained during reduction half-reaction.
Balance the redox reaction ion-electron method:
Cl2+OH−→Cl−+ClO−3 (basic medium)
View SolutionBalancing redox reaction by ion-electron method
Chemical reactions which involve oxidation and reduction (redox reactions) can also be balanced by using the ion-electron method or half-equation method. This method of balancing redox reactions is based upon the principle that electrons lost during oxidation halfreaction of any redox reaction are equal to the electrons gained during reduction half-reaction.
Balance the redox reaction ion-electron method:
Zn+NO−3→Zn2++NH+3 (acidic medium)
View SolutionBalancing redox reaction by ion-electron method
Chemical reactions which involve oxidation and reduction (redox reactions) can also be balanced by using the ion-electron method or half-equation method. This method of balancing redox reactions is based upon the principle that electrons lost during oxidation halfreaction of any redox reaction are equal to the electrons gained during reduction half-reaction.
Balance the redox reaction ion-electron method:
Br2+H2O2→BrO−3+H2O (acidic medium)
View Solution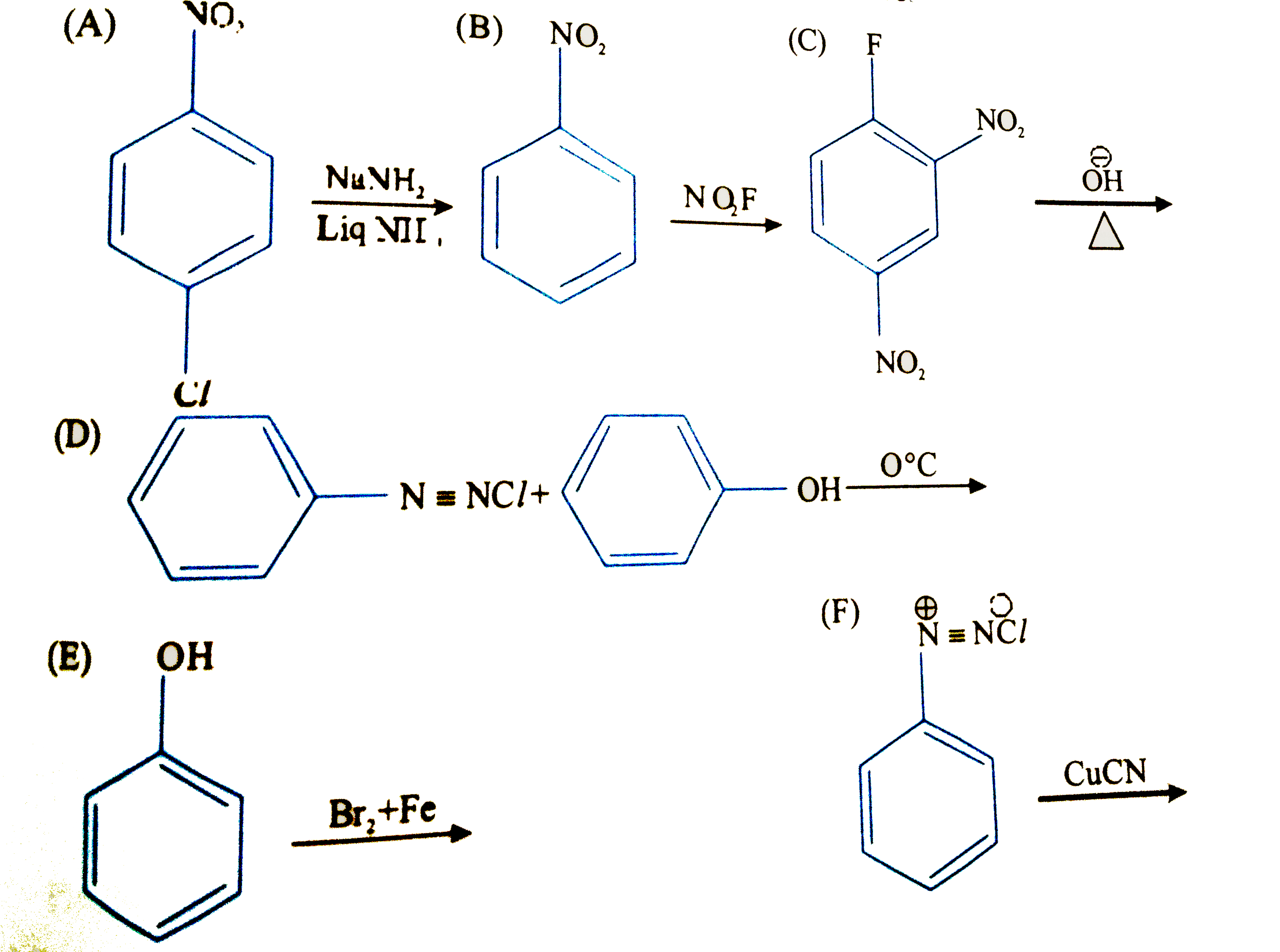
Find out the number of reactions that are electrophilic aromatic substitution aromatic substitution in nature.
View Solution