A monocarboxylic acid decolourise Br2−H2O, on heating with soda lime derivate of styrene is formed , with neutral FeCl3 , a buff coloured precipitate is formed . Acid could be :
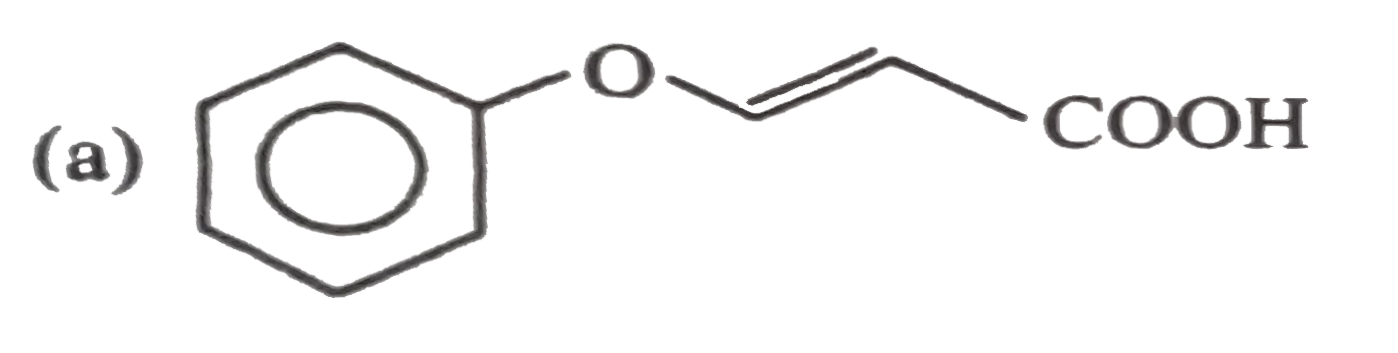
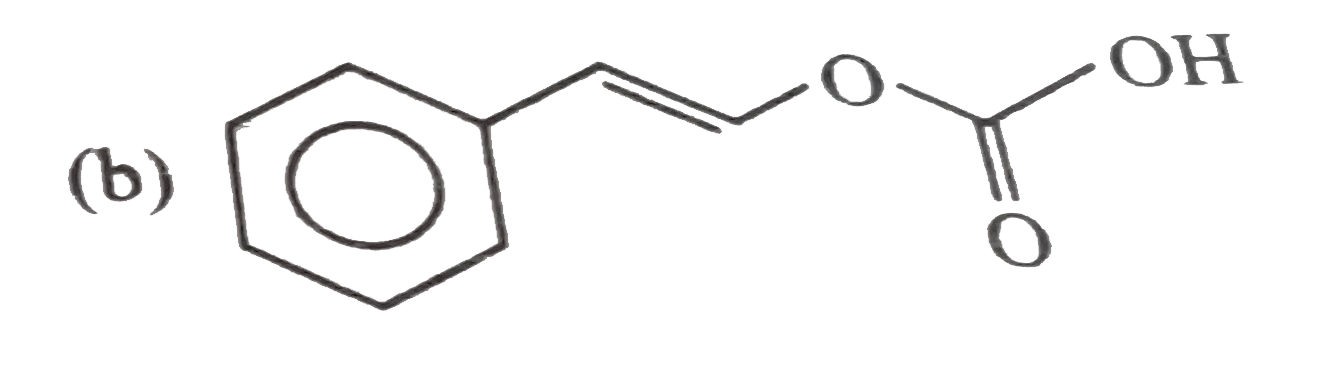
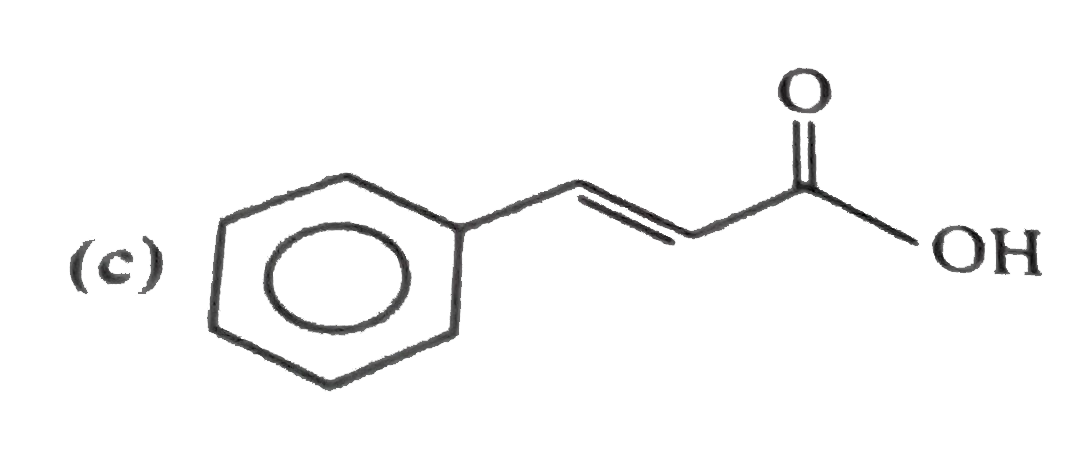
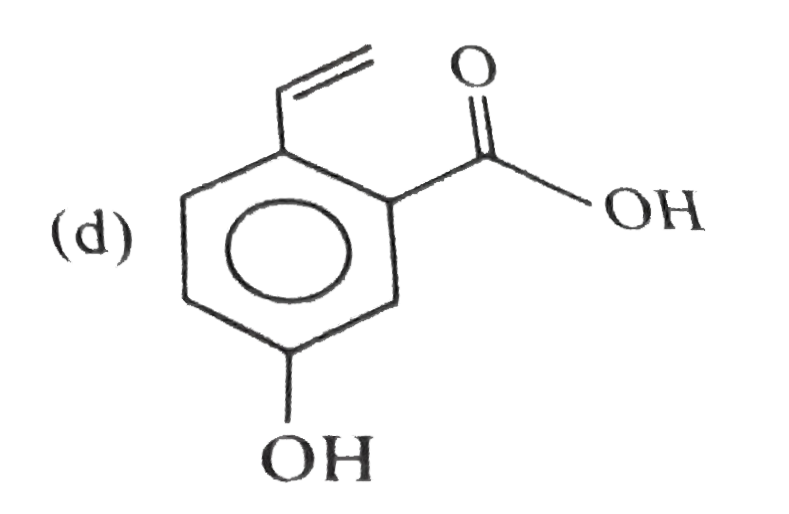
The correct Answer is:D
Step by step video, text & image solution for A monocarboxylic acid decolourise Br_(2) - H_(2)O, on heating with soda lime derivate of styrene is formed , with neutral FeCl_(3) , a buff coloured precipitate is formed . Acid could be : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PRACTICAL ORGANIC CHEMISTRYBook:HIMANSHU PANDEYChapter:PRACTICAL ORGANIC CHEMISTRYExercise:More Than One Correct
Explore 15 Videos
- PRACTICAL ORGANIC CHEMISTRYBook:HIMANSHU PANDEYChapter:PRACTICAL ORGANIC CHEMISTRYExercise:Linked Comprehension Type
Explore 7 Videos
- POLYMERSBook:HIMANSHU PANDEYChapter:POLYMERSExercise:Integer Answer Type Problems
Explore 8 Videos
Similar Questions
Which of the following aromatic compounds will react with KMnO4?
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The most probable structure of compound A is :
View SolutionThe number of condensation copolymers among the following is_________.
SBR, polyester, bakelite, nylon-6, PVC, starch, nylon-6,6, glyptal, natural rubber.
View SolutionSalicylic acid on heating with soda lime gives
View SolutionWhich of the following compounds decolourise Br2−H2O and also give positive test with neutral FeCl3 :
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The most probable structure of compound A is :
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The structure of compound (B) would be :
View SolutionWhich of the following compounds decolourise Br2−H2O and also give positive test with neutral FeCl3 :
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The most probable structure of compound A is :
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The structure of compound (B) would be :
View SolutionWhich acid will not form hydrocarbon on soda lime decarboxylation ?
View SolutionWhich acid gives wine red colour with neutral FeCl3
View SolutionBenzoic acid when heated with soda lime yields
View Solutionसैलिसिलिक अम्ल सोडा-लाइम के साथ गर्म करने पर देता है:
View SolutionBenzoic acid, when heated with soda lime gives ______
View Solutionसेलिसिलिक अम्ल को सोडा लाइम के साथ गर्म करने पर बनने वाला उत्पाद है-
View Solution
