The orbitals having lower energy in tetrahedral complexs according to CFT are
dxy,dyz,dz2
dxy,dyz,dx2−y2
dxy,dyz,dzx
dx2−y2,dz2
The correct Answer is:4
In tetrahedral complexes ligands approach in between the axis.
Similar Questions
Knowledge Check
The tetrahedral complexes are
ANi(CO)4B[FeCl4]−1C[MnCl4]2−D[CoCl4]2−Nearly all tetrahedral complexes are
Ahigh spinBlow spinCintermediate spinDcomplex spinTetrahedral complexes are generally favoured :
Awhere the ligands are bulkyBwhen the ligands are strongerCwhere the electronic configuration of the central metal is d0d5 or d10 (with weak field ligands) as there is no CSFE.Dwhen the central metal ion has pseudo noble gas electron configuration, i.e.(n−1)d10ns0np0.
The splitting diagram for square planar complexes is more complex than for octahedral and tetrahedral complexes and is shown below with the relative energies of each orbital.
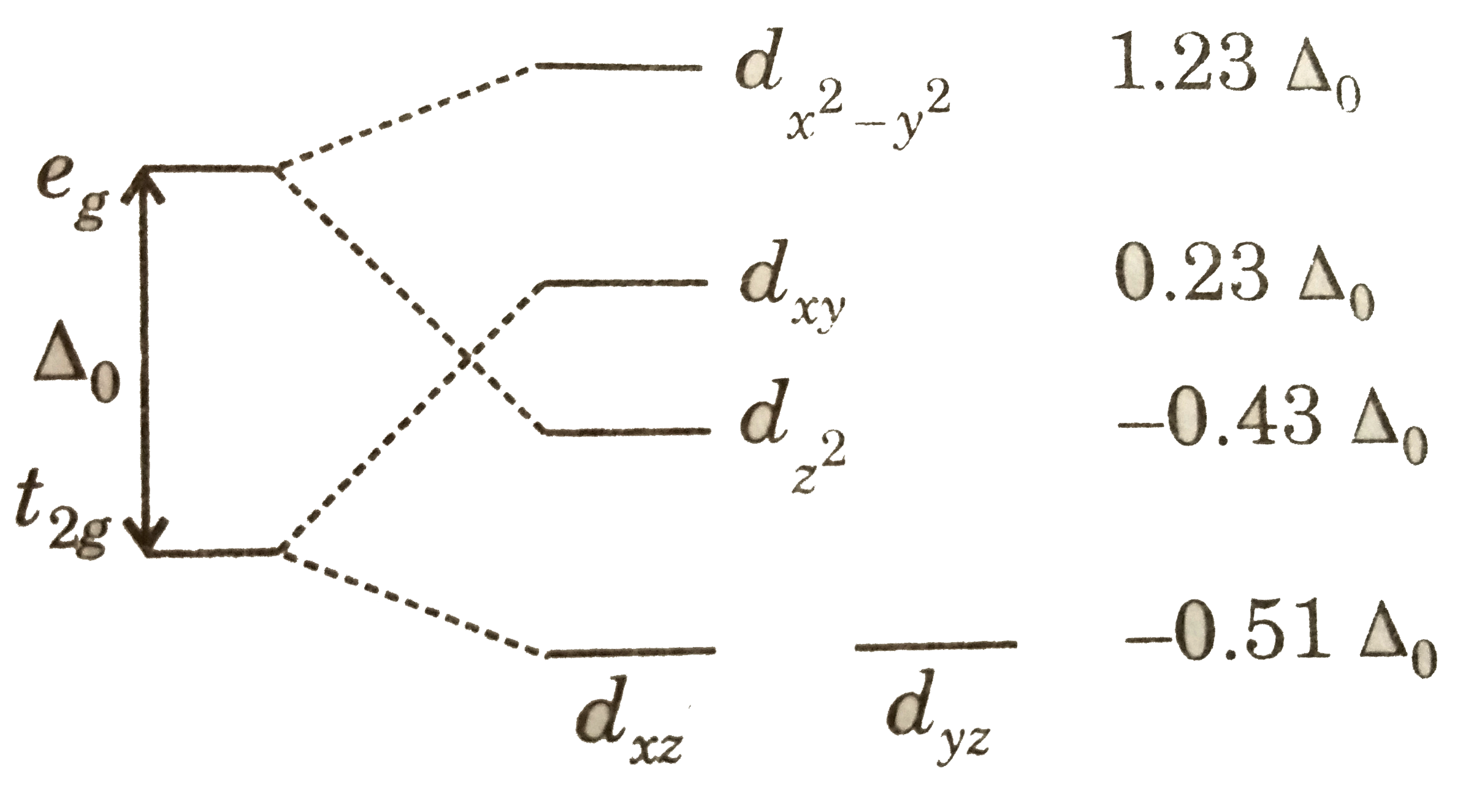
Calculate crystal field stabilisation energy for a diamagnetic square planar d8 metal complex with the help of above diagram neglecting pairing energy (P)
View SolutionTetrahedral complexes are generally favoured :
View SolutionColourless tetrahedral complexes among the following are
View SolutionTetrahedral complexes are generally favoured:
View SolutionSTATEMENT-1: Tetrahedral complexes are always high spin complexes .
STATEMENT-2: Crystal field splitting energy in tetrahedral complexes is 2/3 of the Δ0 (crystal field splitting energy in octahedral complexes).
STATEMENT-3: Tetrahedral complex [MABCD]±n is optically active .
View Solution

