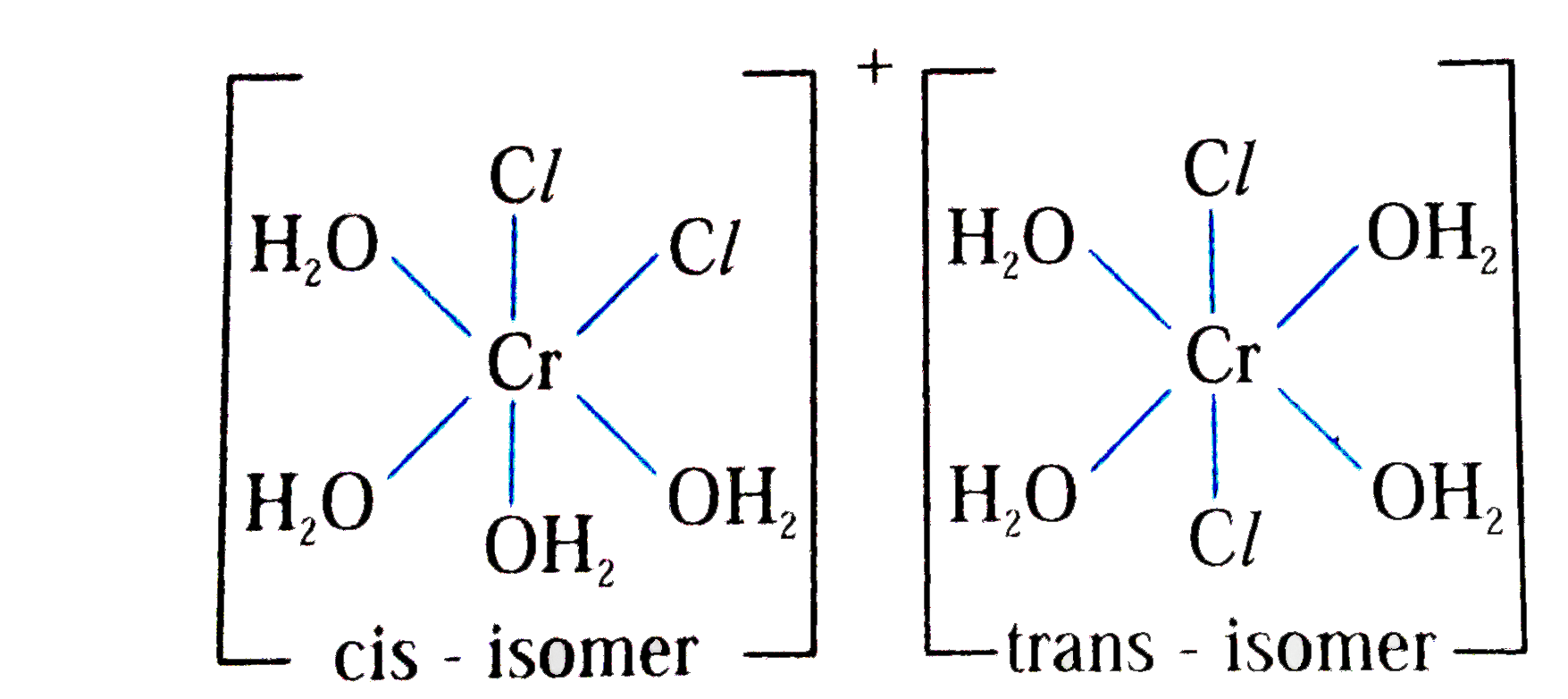
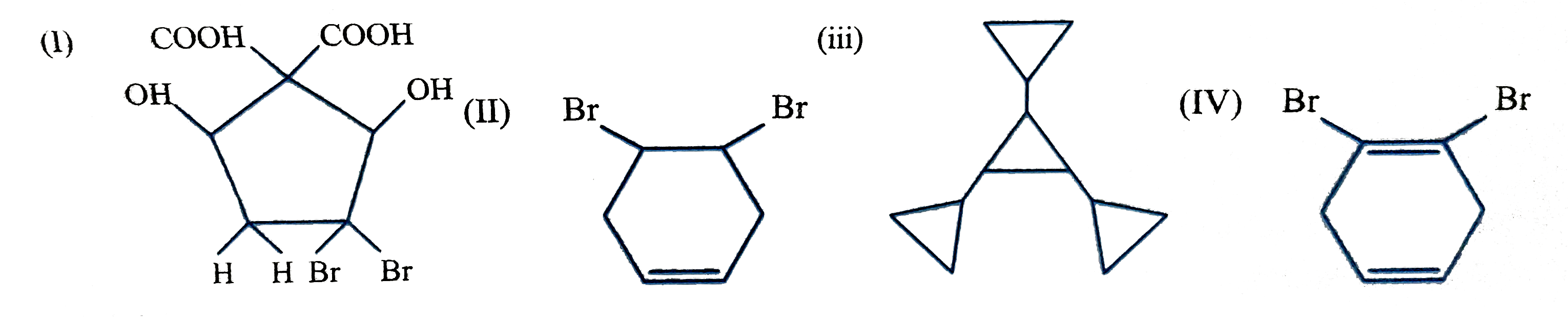
Indicate the complex ion which shows geometrical isomerism.
A
[Cr(H2O)4Cl2]+
B
[Pt(NH3)3Cl]+
C
[Co(NH3)6]3+
D
[Co(CN)5(NC)]3−
Video Solution
Text Solution
The correct Answer is:1
|
Topper's Solved these Questions
- COORDINATION COMPLEXESBook:NARAYNAChapter:COORDINATION COMPLEXESExercise:LEVEL-I (H.W)
Explore 21 Videos
- COORDINATION COMPLEXESBook:NARAYNAChapter:COORDINATION COMPLEXESExercise:LEVEL-II (H.W)
Explore 20 Videos
- COORDINATION COMPLEXESBook:NARAYNAChapter:COORDINATION COMPLEXESExercise:PREVIOUS AIEEE QUESTIONS
Explore 5 Videos
- CO-ORDINATE COMPOUNDS Book:NARAYNAChapter:CO-ORDINATE COMPOUNDS Exercise:Exercise-4
Explore 60 Videos
- D - BLOCK ELEMENTSBook:NARAYNAChapter:D - BLOCK ELEMENTSExercise:CHECK YOUR GRASP
Explore 7 Videos
Similar Questions
Knowledge Check
Indicate the complex ion which shows geometrical isomerism.
A[Cr(H2O)4)Cl2]+B[Pt(NH3)3Cl]C[Pt(NH3)6]3+D[CO(CN)5(NC)]3−Which show geometrical isomerism?
A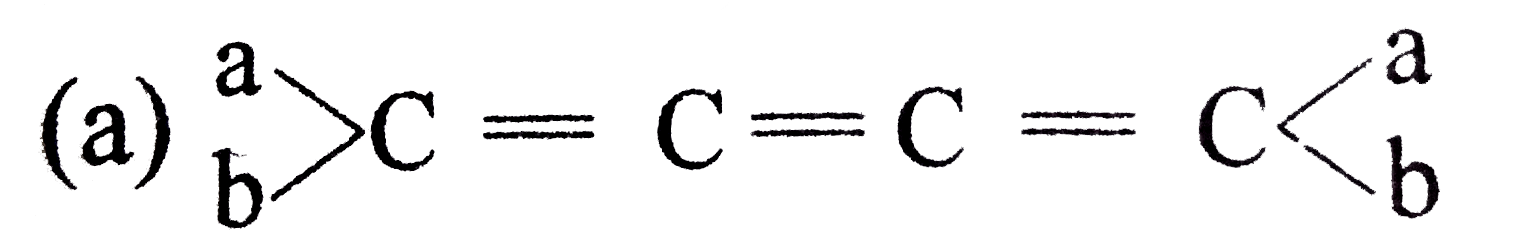 B
B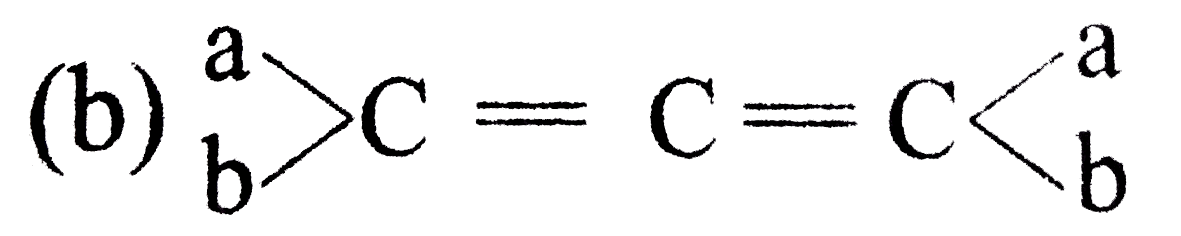 C
C DNone of these
DNone of theseSelect the complex ion which shows geometrical isomerism.
A[Pt(NH3)3Cl]+B[Cr(H2O)4Cl2]+C[Co(NH3)6]3+D[Co(CN)5(NH)]3−
Which will show geometrical isomerism?
View SolutionWhich will show geometrical isomerism?
View SolutionWhich will show geometrical isomerism?
View SolutionWhich will show geometrical isomerism?

View SolutionWhich of the following complex or the complex ion will show geometrical isomerism?
View Solution