From Elligham diagram the correct statements
(a) 4cu+O2→2Cu2O
(b)2C+O2→2CO
( c)2Zn+O2→2ZnO
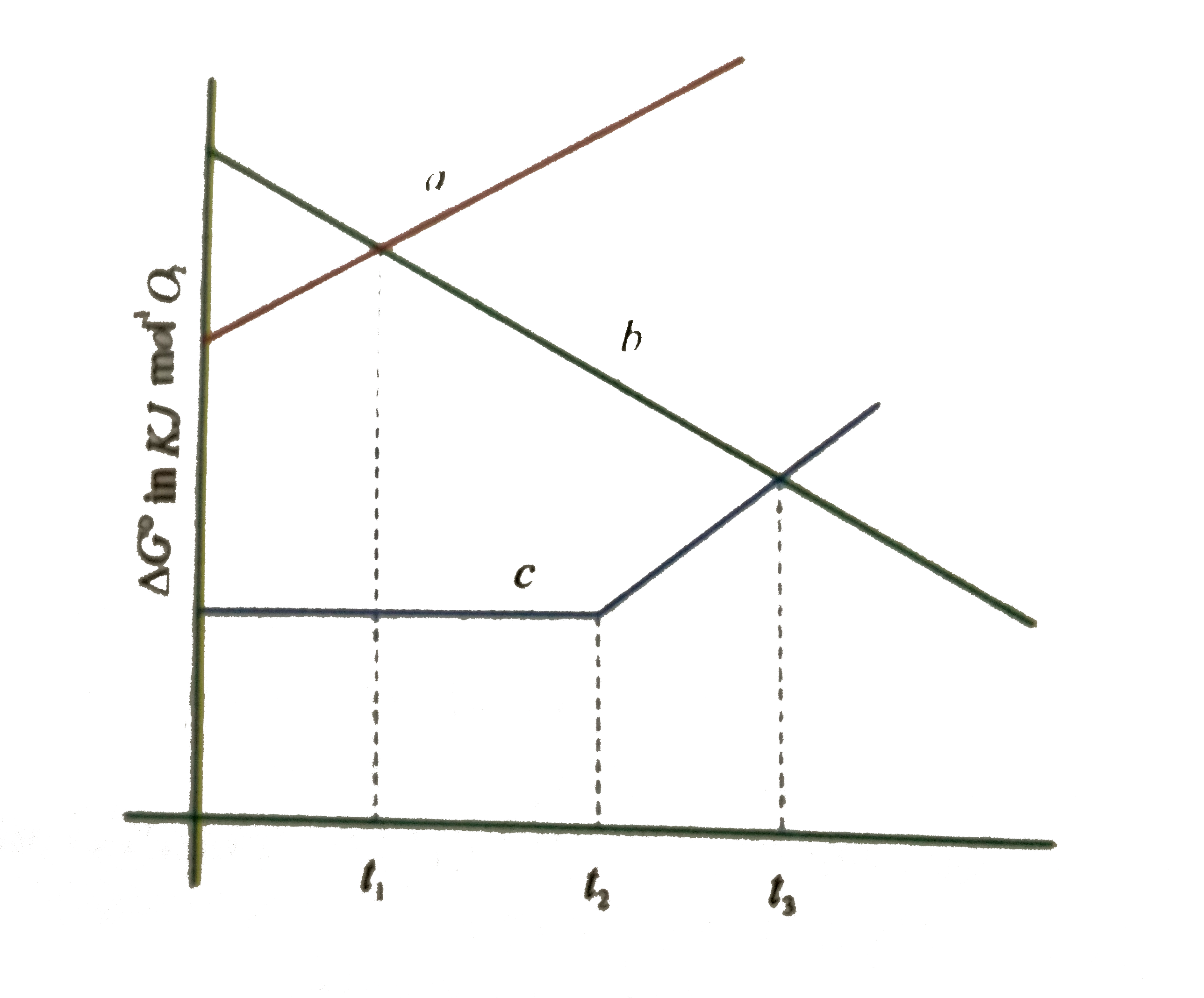
At temeperature above t01C "Carbon" can reduce Cu2O
At temeperature above t03C "Carbon" can reduce ZnO
Reduction of Cu2O with carbon requires high temperature when compared with the reduction of ZnO by carbon
All
The correct Answer is:1
Similar Questions
Knowledge Check
For the reaction,
C+O2→CO2,ΔH=−393J
2Zn+O2→2ZnO,H=−412JACarbon reduce ZnO to ZnBOxidation of carbon is not feasibleCOxidation of Zn is not feasibleDZn liberates more heat than carbon during oxidationIn C+H2O→CO+H2, H2O acts as
AOxidising agentBReducing agentC(a) and (b) bothDNone of theseC+O2→CO2,
CO+12O2→CO2,
Then ΔfH forCO will beA2X+YBX−YCY−2XDY−X
For which one of the following reaction,the graph of ΔG against T is almost horizontal to temperature axis
(А) C(s) + O2(g) → CO2(g)
(В) 2C(s) + O2(g) → 2CO(g)
(с) 2CO(s) + O2(g) → 2CO2(g)
(D) 2Mg(s) + O2(g) → 2MgO(s)
View SolutionComplete the following reactions
(i) O2−2+H2O→ (ii) O−2+H2O→
View SolutionFind the formula of A
ZnS+O2→(A)+SO2.
View SolutionFor the reaction, C+O2→CO2, ΔH=−393J
2Zn+O2→2ZnO, ΔH=−412J
View SolutionWith respect to halogen or iodide ion four reactions are give below
(P) Cl2+H2O2→2HCl+O2
(Q) Br2+H2O2→2HBr+O2
( R) I2+H2O2→2HI+O2
(S) I−+H2O2→2OH−+I2
The correct reactions are :
View Solution

