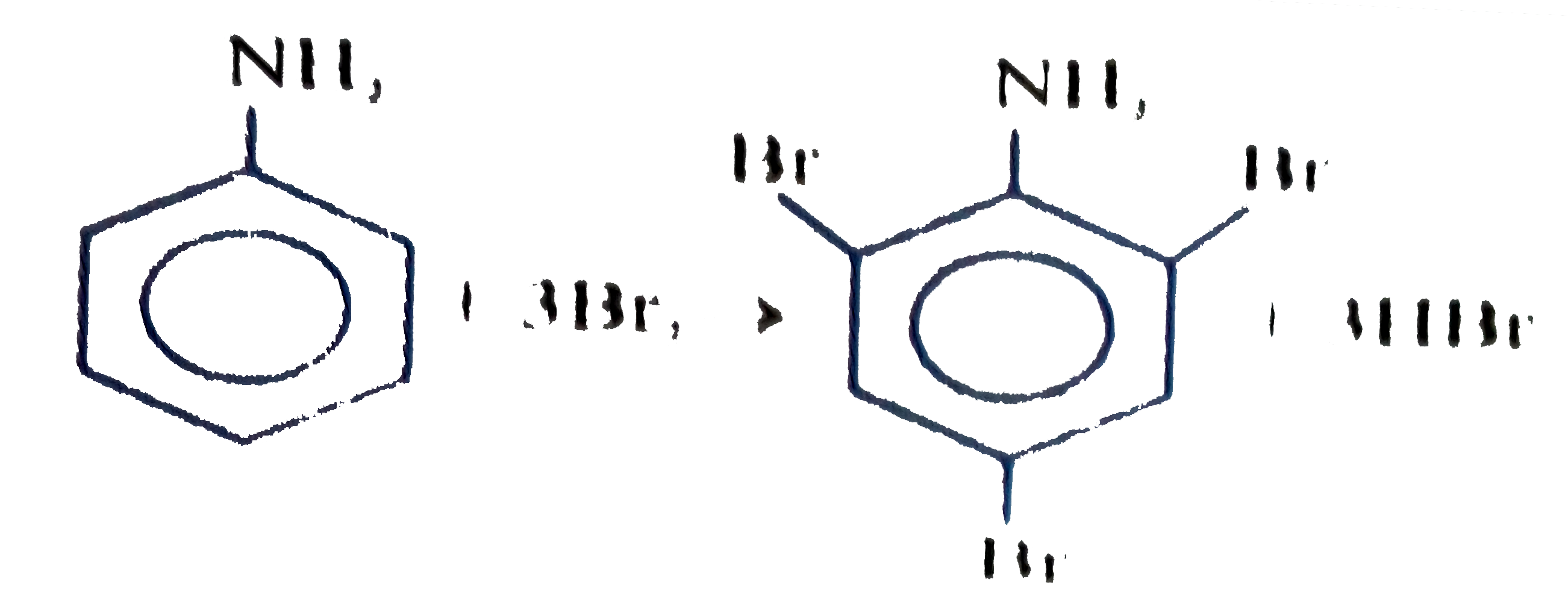
Aniline (1 mole) react with bromine to give symmetrical tribromoanline. The amount of bromine required is
A
3.0 moles
B
1.5 moles
C
4.5 moles
D
6.0 moles
Video Solution
Text Solution
The correct Answer is:A
|
Topper's Solved these Questions
- AMINES & DIAZO COMPOUNDSBook:NARAYNAChapter:AMINES & DIAZO COMPOUNDSExercise:Previous year
Explore 9 Videos
- AMINES & DIAZO COMPOUNDSBook:NARAYNAChapter:AMINES & DIAZO COMPOUNDSExercise:Level -III
Explore 19 Videos
- AMINES & DIAZO COMPOUNDSBook:NARAYNAChapter:AMINES & DIAZO COMPOUNDSExercise:Level -I (C.W)
Explore 21 Videos
- ALDEHYDES, KETONES & CARBOXYLIC ACIDSBook:NARAYNAChapter:ALDEHYDES, KETONES & CARBOXYLIC ACIDSExercise:CHECK YOUR GRASP
Explore 6 Videos
- BIOMOLECULESBook:NARAYNAChapter:BIOMOLECULESExercise:EXERCISE-4 (NCERT EXAMPLAR PROBLEMS)
Explore 12 Videos
Similar Questions
Knowledge Check
Phenol (1 mole ) reacts with bromine to give tribromophenol . The amount of bromine required is
A3.0 moleB1.5 moleC4.5 moleD6.0 moleBenzoic acid reacts with bromine to give
Ao−bromobenzoic acidBm−bromobenzoic acidCp−bromobenzoic acidD3, 4-dibromobenzoic acid.Phenol reacts with aqueous bromine to give :
Ao - bromophenolBm - bromophenolCo & p - bromophenolD2, 4, 6 - tribromophenol.
Bromine water reacts with aniline to give
View SolutionBromine water reacts with aniline to give
View SolutionWhen phenol reacts with bromine in CS2 at a low temperature, the product is :
View SolutionPhenol reacts with bromine is CS2 to give :
View SolutionSilver benzoate reacts with bromine to form
View Solution