Z' in the following sequence of reaction is C6H6HNO3/H2SO4−−−−−−−−→ΔWZn/HCl−−−−→XNaNO2−−−−−−−→HCl,0−5∘CYH2∅H3PO2−−−−−→Z

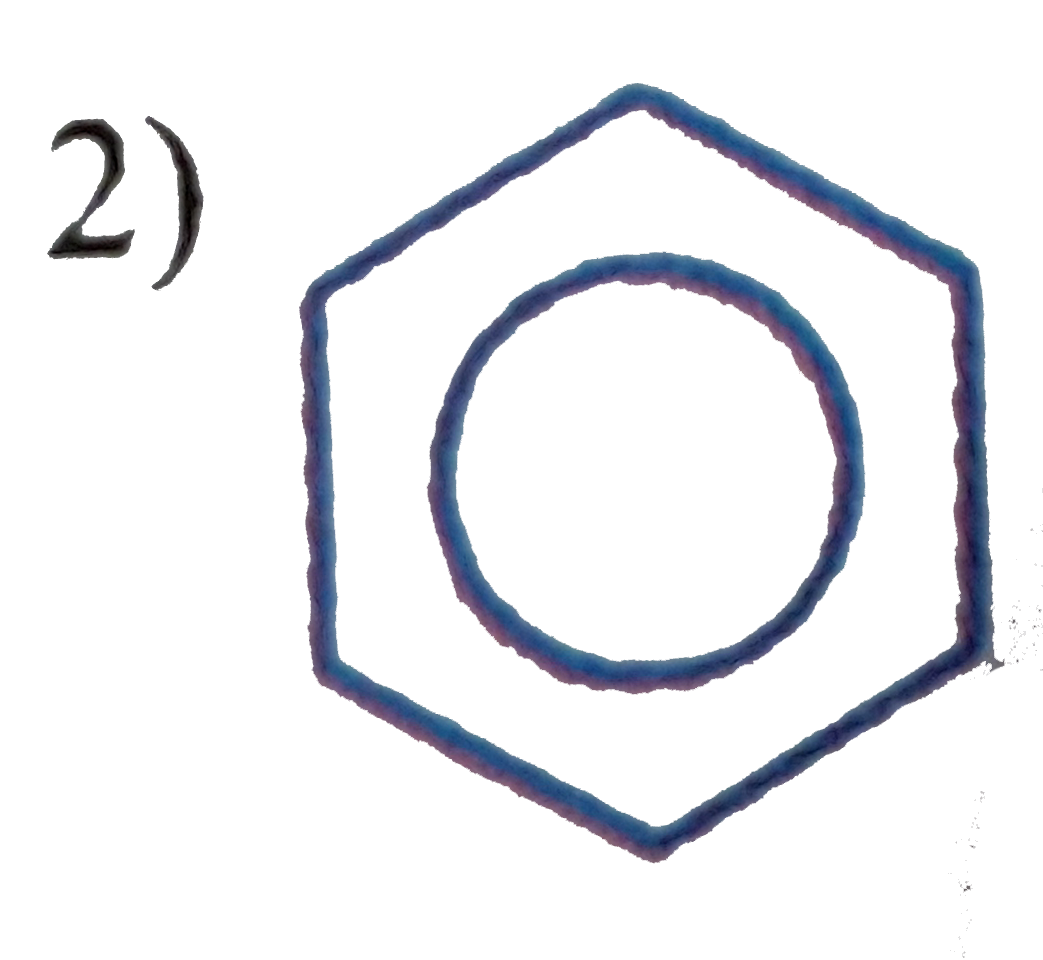
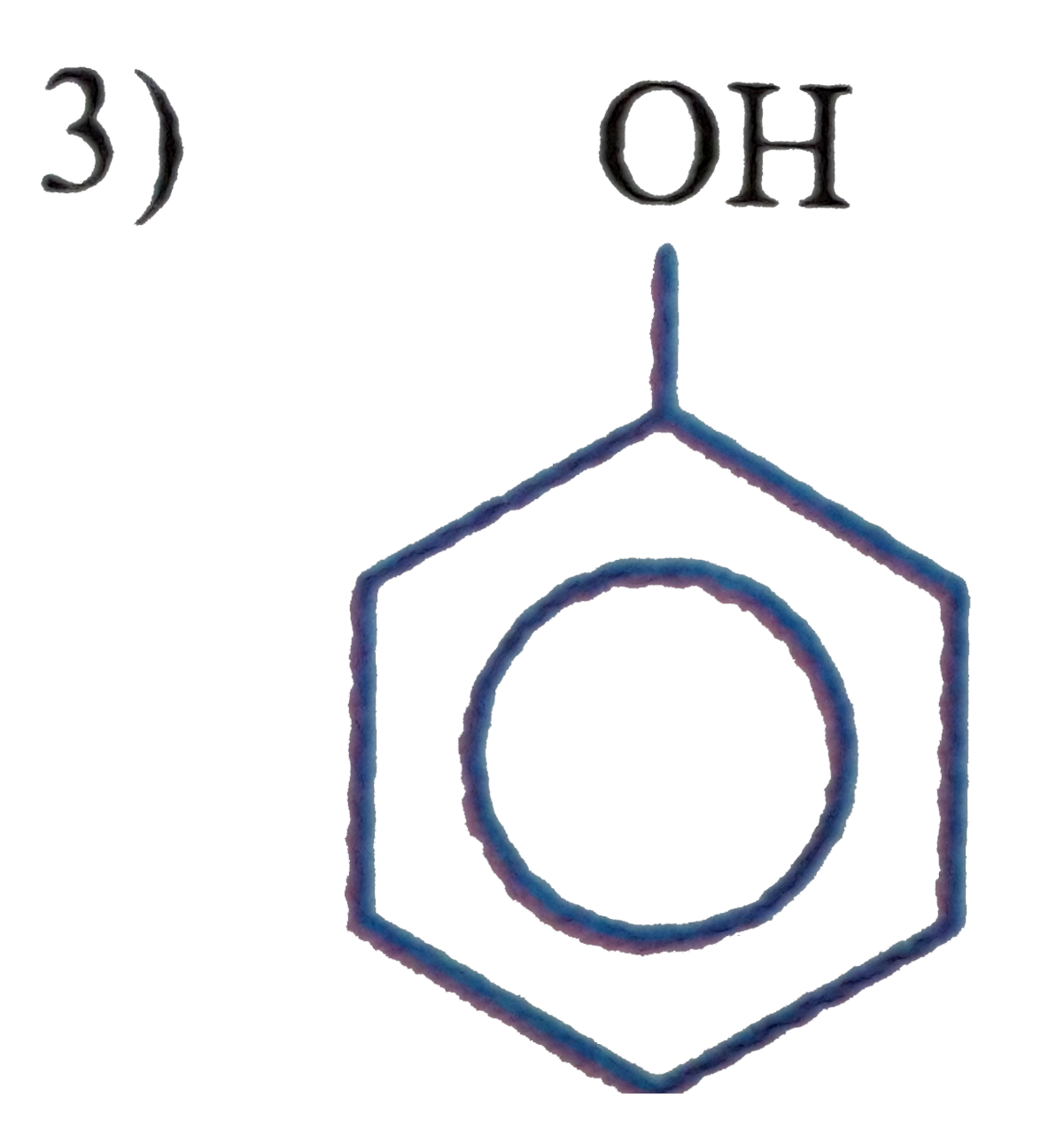
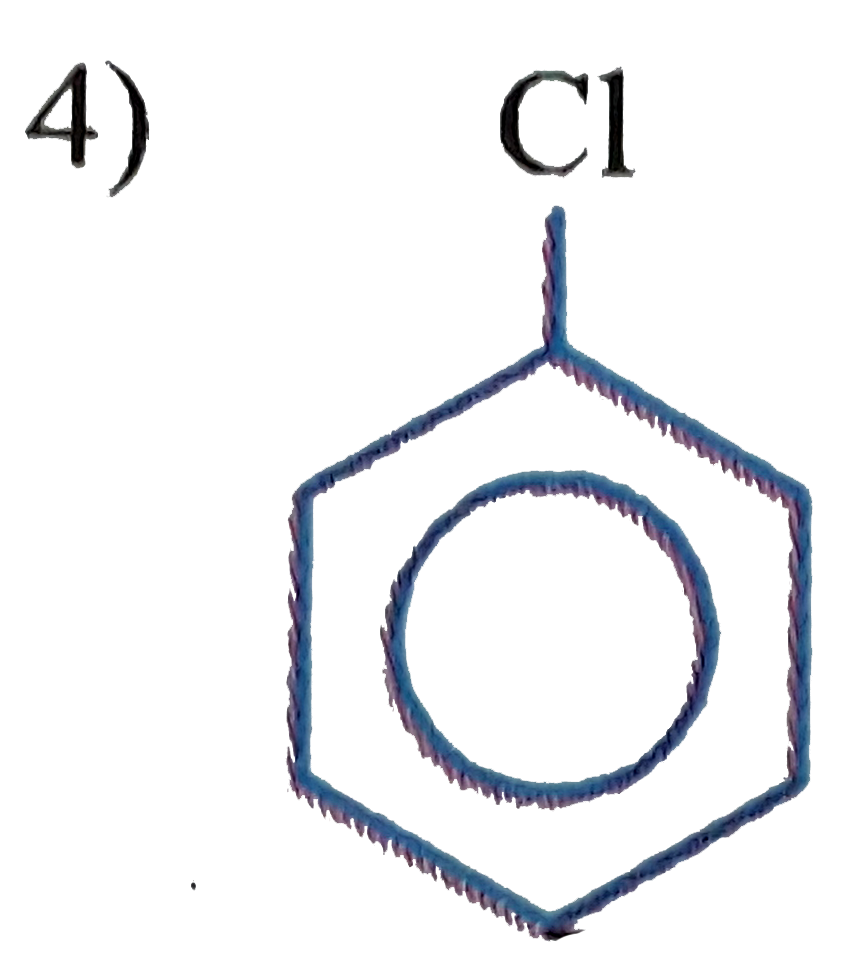
The correct Answer is:B
To solve the problem step by step, we will analyze each reaction in the sequence provided and determine the final product Z.
Step 1: Nitration of Benzene
- Reaction: C6H6HNO3ΔH2SO4
- Process: Benzene reacts with a mixture of nitric acid (HNO₃) and sulfuric acid (H₂SO₄) to undergo nitration. The electrophile generated in this reaction is the nitronium ion (NO₂⁺).
- Product W: The product formed is nitrobenzene (C6H5NO2).
Step 2: Reduction of Nitrobenzene
- Reaction: WZn/HCl→
- Process: Nitrobenzene undergoes reduction when treated with zinc (Zn) and hydrochloric acid (HCl). The nitro group (–NO₂) is reduced to an amino group (–NH₂).
- Product X: The product formed is aniline (C6H5NH2).
Step 3: Diazotization of Aniline
- Reaction: XNaNO2→HCl,0−5∘C
- Process: Aniline reacts with sodium nitrite (NaNO₂) in the presence of hydrochloric acid (HCl) at low temperatures (0-5°C). This reaction forms diazonium salt.
- Product Y: The product formed is benzene diazonium chloride (C6H5N2Cl).
Step 4: Hydrolysis of Benzene Diazonium Chloride
- Reaction: YH2O/H3PO2→
- Process: Benzene diazonium chloride undergoes hydrolysis when treated with water and phosphorous acid (H₃PO₂). The diazonium group (–N₂⁺) is replaced by a hydrogen atom (–H).
- Product Z: The final product formed is benzene (C6H6).
Final Answer
Thus, the final product Z in the sequence of reactions is benzene (C6H6).
---
- Reaction: C6H6HNO3ΔH2SO4
- Process: Benzene reacts with a mixture of nitric acid (HNO₃) and sulfuric acid (H₂SO₄) to undergo nitration. The electrophile generated in this reaction is the nitronium ion (NO₂⁺).
- Product W: The product formed is nitrobenzene (C6H5NO2).
- Reaction: WZn/HCl→
- Process: Nitrobenzene undergoes reduction when treated with zinc (Zn) and hydrochloric acid (HCl). The nitro group (–NO₂) is reduced to an amino group (–NH₂).
- Product X: The product formed is aniline (C6H5NH2).
- Reaction: XNaNO2→HCl,0−5∘C
- Process: Aniline reacts with sodium nitrite (NaNO₂) in the presence of hydrochloric acid (HCl) at low temperatures (0-5°C). This reaction forms diazonium salt.
- Product Y: The product formed is benzene diazonium chloride (C6H5N2Cl).
- Reaction: YH2O/H3PO2→
- Process: Benzene diazonium chloride undergoes hydrolysis when treated with water and phosphorous acid (H₃PO₂). The diazonium group (–N₂⁺) is replaced by a hydrogen atom (–H).
- Product Z: The final product formed is benzene (C6H6).
Thus, the final product Z in the sequence of reactions is benzene (C6H6).
Similar Questions
Knowledge Check
Z' in the following sequence of reactions is
C6H6HNO3/H2SO4−−−−−−−−→ΔWZn/HCl−−−−→XNaNO2−−−−→HClYH2∅H3PO2−−−−−→ZA B
B C
C D
D
For the sequence of reactions,
RC6H5NO2HNO3−−−→H2SO4A(NH4)2S−−−−−→B, the compound BisAAnilineBm- dinitrobenzeneCm- NitroanilineDm- diaminobenzeneThe final product in the following sequence of ractions is C6H5NH2NaNO2/HCl−−−−−−−→273−278KAC6H6/NaOH−−−−−−−−→B
AC6H5N2ClBC6H5OHCC6H5−C6H5DC6H5N=NOH
In the sequence of reaction: C6H5NH2NaNO2−−−−→HClXH3PO2−−−→Cu+Y. The product Y is:
View SolutionIdentify C in the following sequence of reactions:
C6H5−NO2Sn+HCl−−−−−→ANaNO2/HCl−−−−−−−→0∘CBH3PO2/H2O−−−−−−−→C
View SolutionConsider the following sequence of reaction.
C6H5NH2NaNO2−−−−→HClXCu2(CN)2−−−−−−→YH2O/H+−−−−−→Z
Z is identified as
View SolutionC6H5−NH2NaNO2/HCl−−−−−−−→0∘CACuCl2/HCl−−−−−−−→B, is
View SolutionA,B and C , in the following reaction sequence C6H660∘C,conc HNO3−−−−−−−−−−−→ concH2SO4
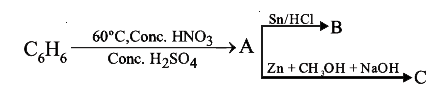
View Solution

