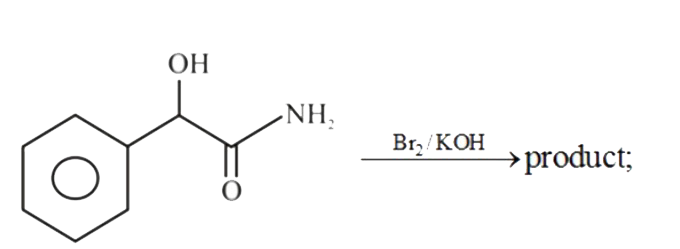
How many mole of NaOH are consumed in Hoffmann Bromamide reaction?
Video Solution
Text Solution
The correct Answer is:D
R−CONH2+Br2+4NaOH→2NaBr+R−NH2+Na2CO3+2H2O
|
Similar Questions
Knowledge Check
R−O∣∣C−NH2+xNaOH+Br2→R−NH2+2NaBr+Na2CO3+H2O
Number of moles of NaOH used in above Hoffmann bromamide reaction is :A3B4C5D6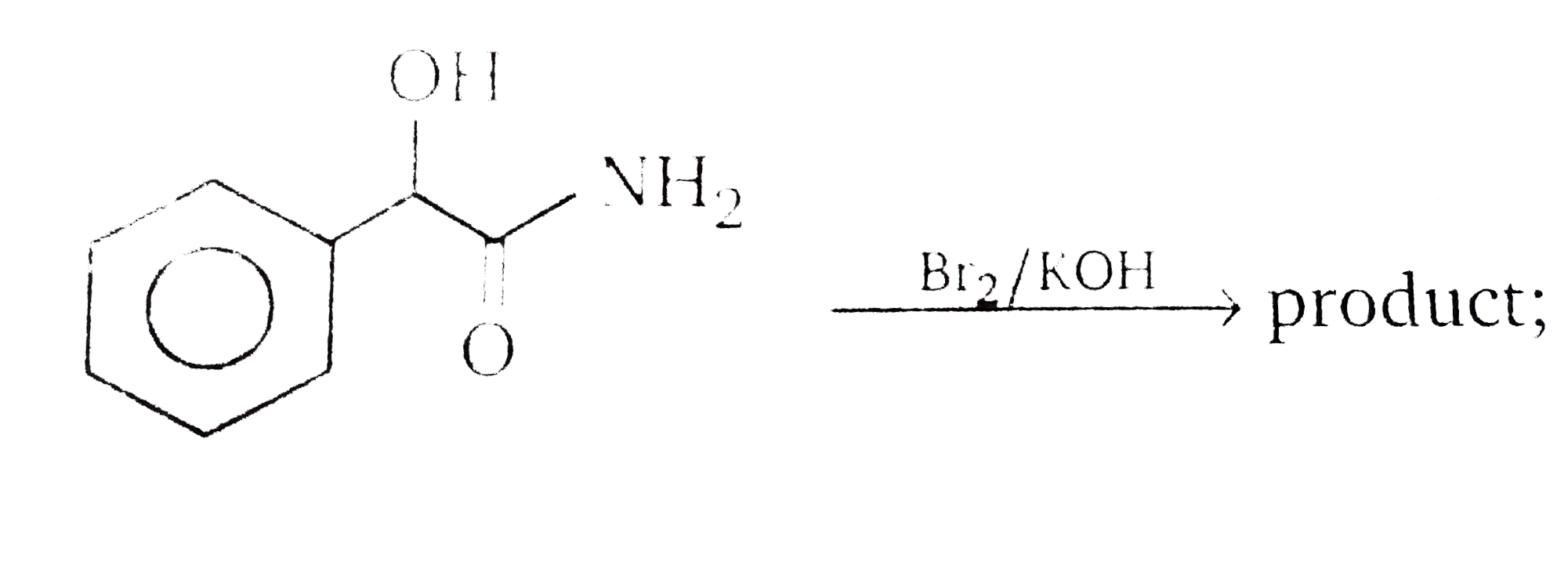
Product of this Hoffmann bromamide reaction is :APh−O∣∣C−CH3BPh - CHOC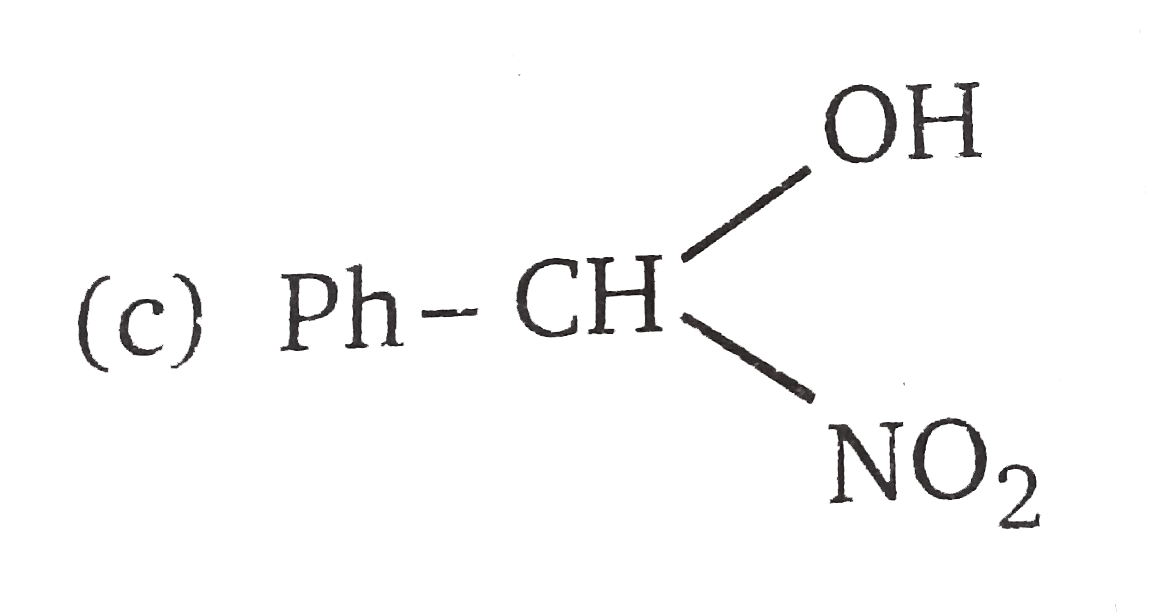 DPh−CH2−NH2
DPh−CH2−NH2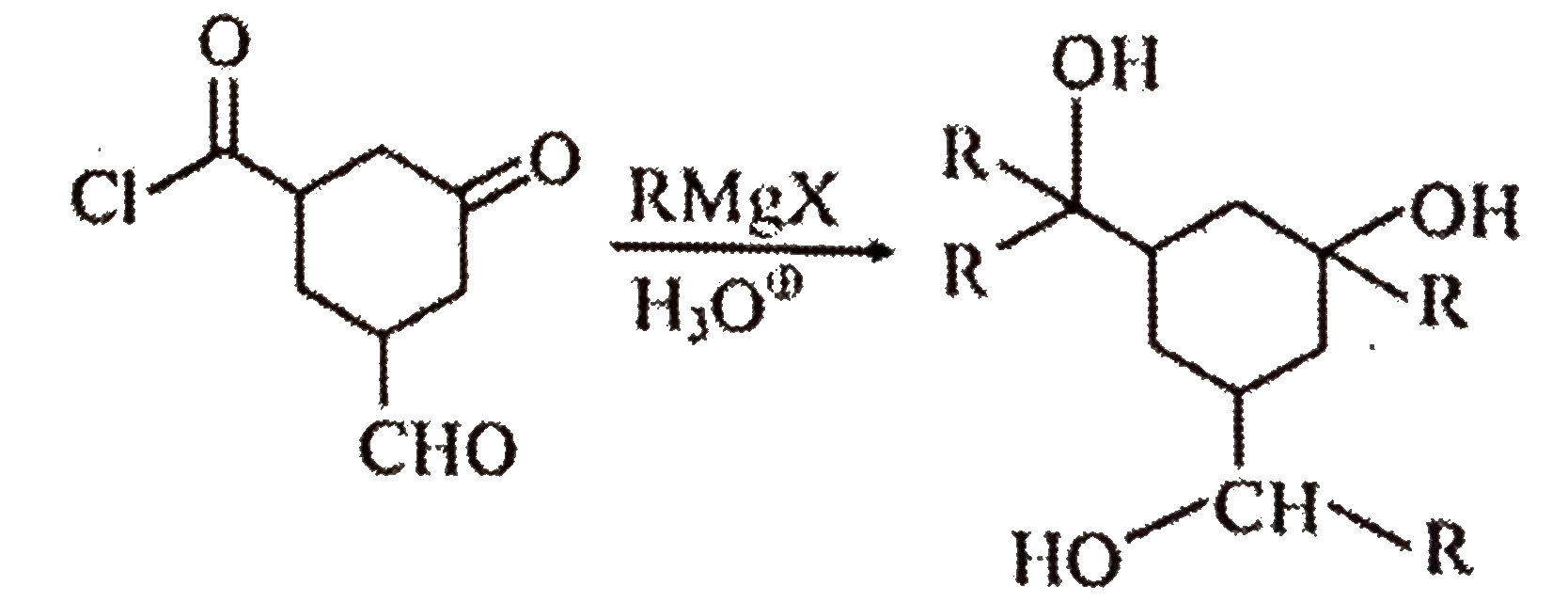
How many molecules of RMgX are consumed in the above given reaction ?A2B4C5D6
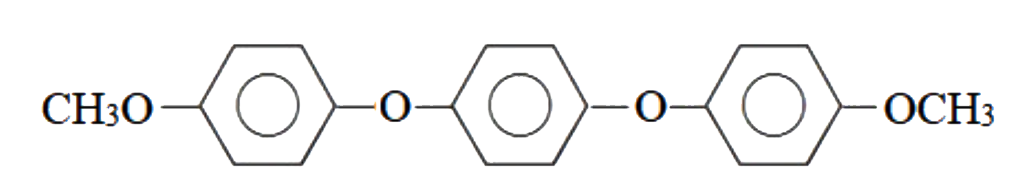
How many moles of HI consumed in above reaction?
View Solution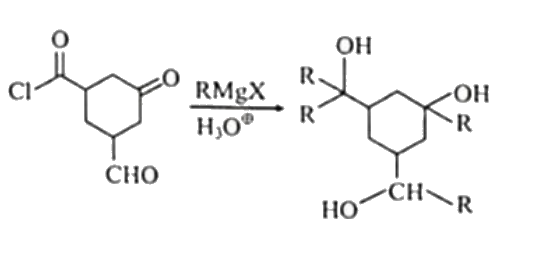
How many molecules of RMgX are consumed in the above given reaction?
View SolutionHoffmann Bromamide Degradation Reaction
View SolutionWrite a short note on Hofmann's bromamide reaction.
View Solution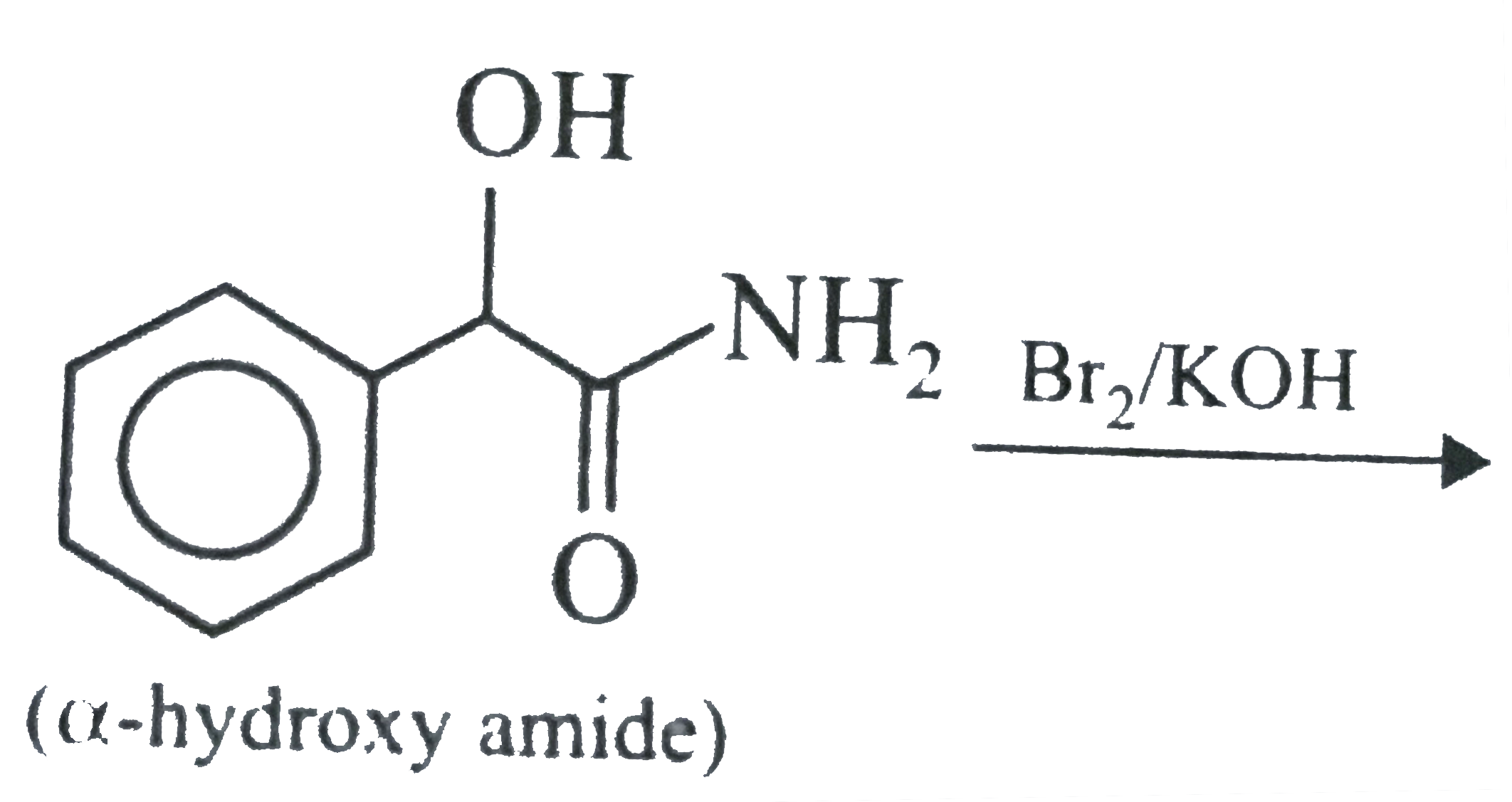
Product of this Hofmann bromamide reaction is
View Solution